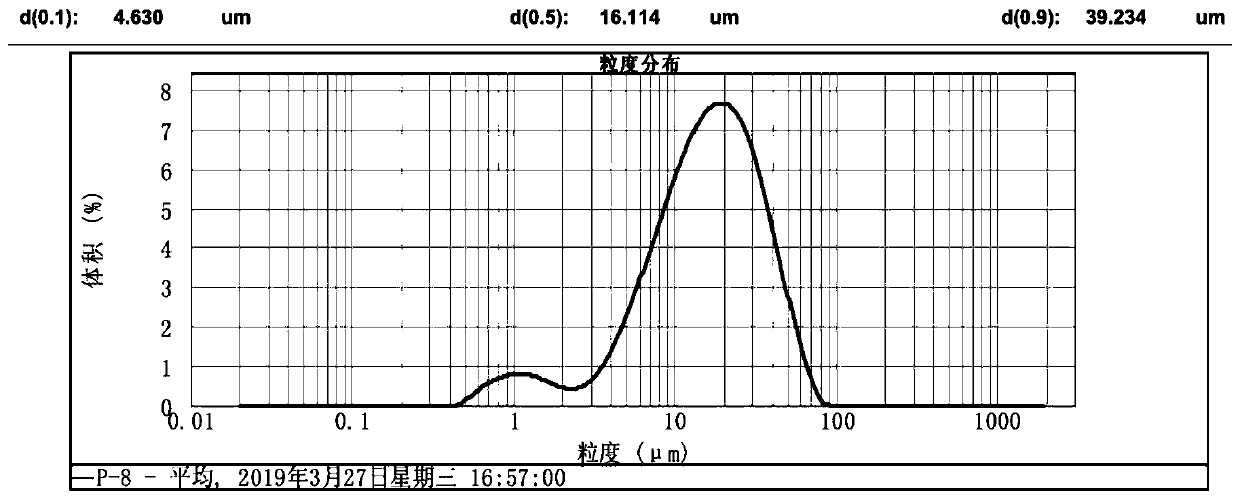
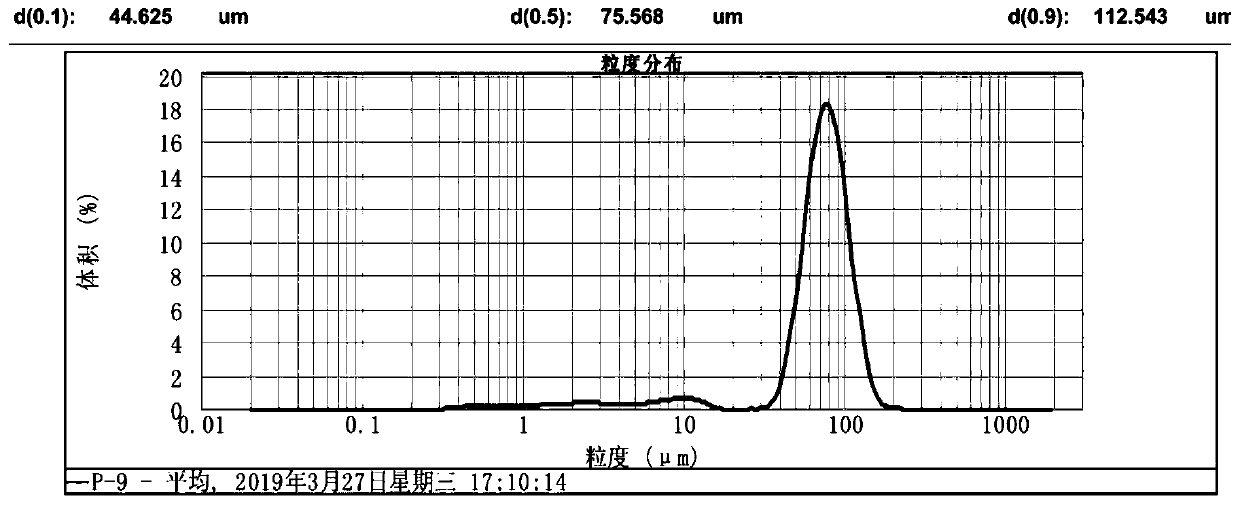
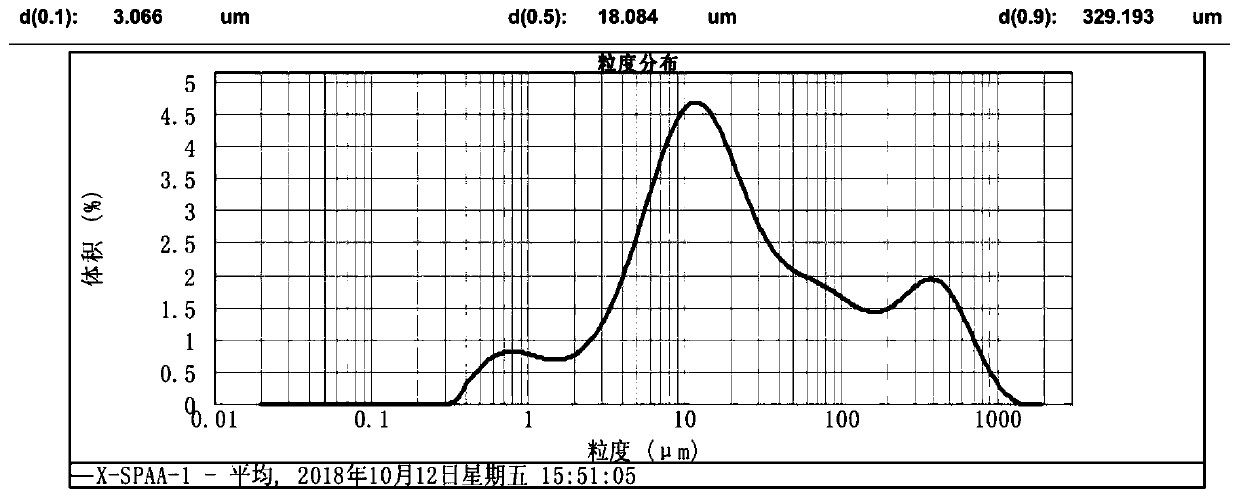
Method for preparing large-particle lithium carbonate from salt lake lithium-rich brine
A technology of large particles and lithium carbonate, which is applied in the direction of lithium carbonate; Effect of improving purity and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] A method for preparing large particles of lithium carbonate from salt lake lithium-rich brine, wherein the content of lithium ions in the lithium-rich brine of the salt lake is 2.52 wt%, the content of potassium ions is 0.25 wt%, the content of magnesium ions is 0.09 wt%, and the content of sodium ions is 0.74 wt%. wt%, the sulfate content is 0.08wt%.
[0067] Include the following steps:
[0068] Step 1: Stir and heat 180 g of salt lake lithium-rich brine to 78°C. After the heating is completed, maintain stirring and temperature and add 7.4 g of surfactant to the salt lake lithium-rich brine. The added mass of the surfactant per minute is the Said salt lake lithium-rich brine quality 0.35%, obtains the salt lake lithium-rich brine after pretreatment;
[0069] Described surfactant is the aqueous solution of polypropylene ammonium salt (molecular weight is 3~100,000 daltons), sodium lauryl sulfate and sodium hexametaphosphate, and the mass of polypropylene ammonium salt...
Embodiment 2
[0081] A method for preparing large particles of lithium carbonate from salt lake lithium-rich brine, wherein the content of lithium ions in the lithium-rich brine of the salt lake is 2.00 wt%, the content of potassium ions is 0.1 wt%, the content of magnesium ions is 0.08 wt%, and the content of sodium ions is 0.20 wt%. wt%, the sulfate content is 0.02wt%.
[0082] Include the following steps:
[0083] Step 1: Stir and heat 180 g of salt lake lithium-rich brine to 70°C. After heating, maintain stirring and temperature and add 10 g of surfactant to the salt lake lithium-rich brine. The mass of surfactant added per minute is the The quality of salt lake lithium-rich brine is 0.2%, and the pretreated salt lake lithium-rich brine is obtained;
[0084] Described surfactant is the aqueous solution of polypropylene ammonium salt (molecular weight is 3~100,000 daltons), sodium lauryl sulfate and sodium hexametaphosphate, and the mass of polypropylene ammonium salt in described surfa...
Embodiment 3
[0089] A method for preparing large particles of lithium carbonate from salt lake lithium-rich brine, wherein the content of lithium ions in the lithium-rich brine of the salt lake is 3.00 wt%, the content of potassium ions is 0.4 wt%, the content of magnesium ions is 0.1 wt%, and the content of sodium ions is 1.00 wt%. wt%, the sulfate content is 0.10wt%.
[0090] Include the following steps:
[0091] Step 1: Stir and heat 180 g of salt lake lithium-rich brine to 85°C. After the heating is completed, maintain stirring and temperature and add 10 g of surfactant to the salt lake lithium-rich brine. The added mass of the surfactant per minute is the The quality of salt lake lithium-rich brine is 0.5%, and the salt lake lithium-rich brine after pretreatment is obtained;
[0092] Described surfactant is the aqueous solution of polypropylene ammonium salt (molecular weight is 3~100,000 daltons), sodium lauryl sulfate and sodium hexametaphosphate, the quality of polypropylene ammon...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com