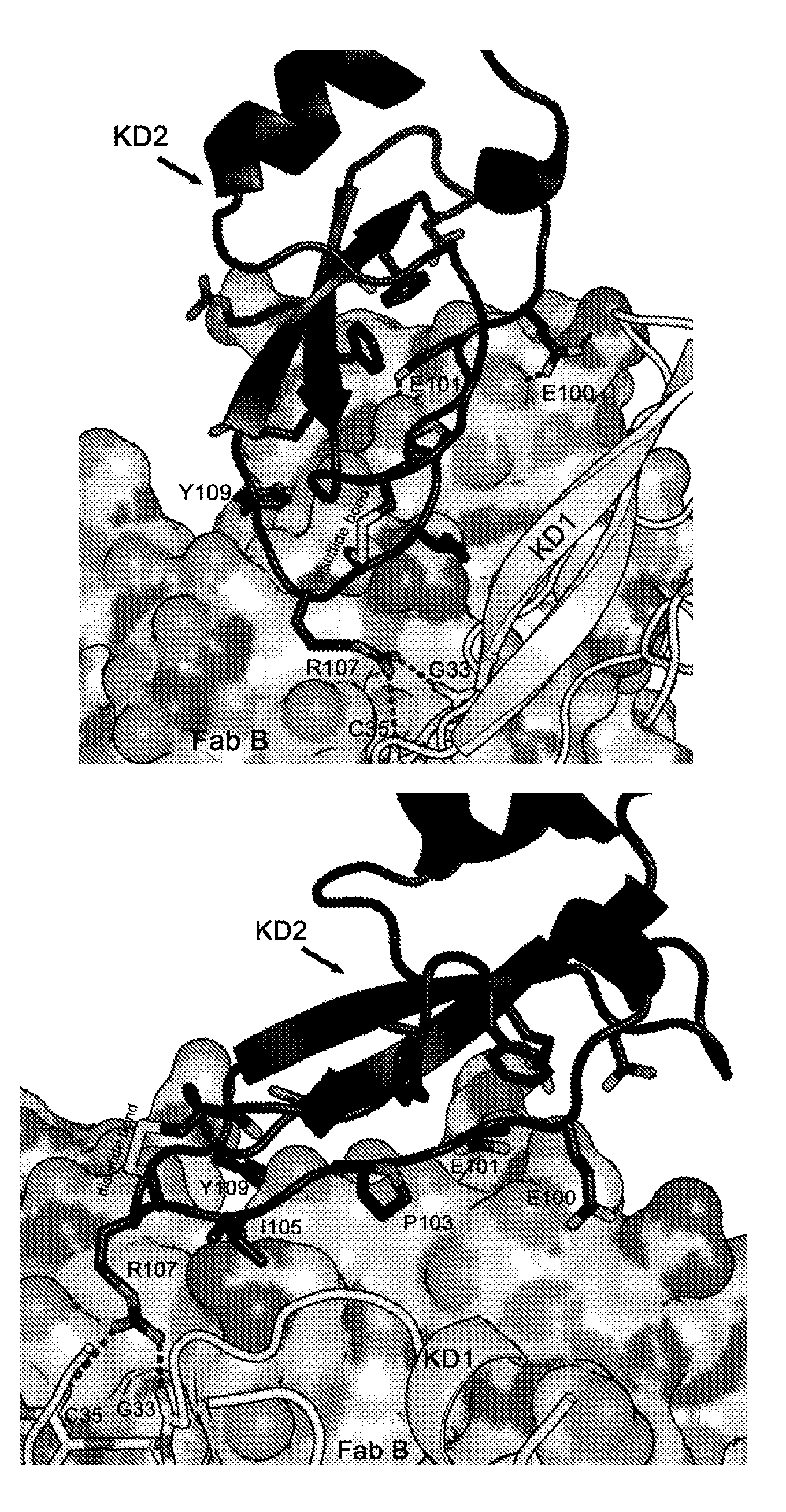Monoclonal antibodies against tissue factor pathway inhibitor (TFPI)
A monoclonal antibody, tissue factor technology, applied in the direction of antibodies, anti-animal/human immunoglobulin, anti-peptide structure protease inhibitor immunoglobulin, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0110] Example 1. Expression and purification of recombinant TFPI (Kunitz domain 2) from Escherichia coli.
[0111] expression system
[0112] A destination vector named pD Eco5 N (according to Gateway nomenclature) was employed. pD Eco5 N is based on pET-16 b (Novagen) and additionally encodes His 10 and NusA tags and a Gateway cloning cassette for expressing fusion proteins composed of His 10 / NusA and target protein composition.
[0113] The TFPI construct encoding the thrombin cleavage site and Gateway binding site (attBl-5#, attB2-3#, Invitrogen) fused to the N-terminus of Kunitz domain 2 (Lys93 to Phe154, see Uniprot 10646) was cloned into the pD Eco5 N vector, resulting in an expression vector designated pD Eco5 N TFPI KD2. The BL21 DE3 (Novagen) expression strain was used.
[0114] Amino acid sequence of the fusion protein expressed using pD Eco5 N TFPI KD2, 600 AA
[0115]
[0116] sequence component
[0117]
[0118] Express
[0119] BL21 DE3 strain t...
Embodiment 2
[0122] Example 2. Production of recombinant monoclonal antibody Fab A against TFPI, expression and purification in Escherichia coli
[0123] Express
[0124] Fab A was co-expressed using expression vector pET28a and E. coli strain BL21 Star DE3. The light and heavy chain regions encoded on the expression vector were fused to the periplasmic signal sequence at their N-termini, respectively. The heavy chain region also encodes His at its C-terminus 6 Tags are used to purify Fabs. Transformed E. coli strains grown overnight in TB-Instant expression medium were used to self-induce recombinant protein expression (#71491, Novagen). Briefly, 10 mL of transformed E. coli culture (in a 50 mL Falcon tube) was grown as a preculture in LB medium with 30 μg / mL kanamycin for 14 h at 37 °C with agitation. The speed is 180 rpm. Subsequently, 4 Erlenmeyer flasks with 500 mL of TB-Instant overnight expression medium were each inoculated with 2 mL of the pre-culture, and incubated at 30 °C ...
Embodiment 3
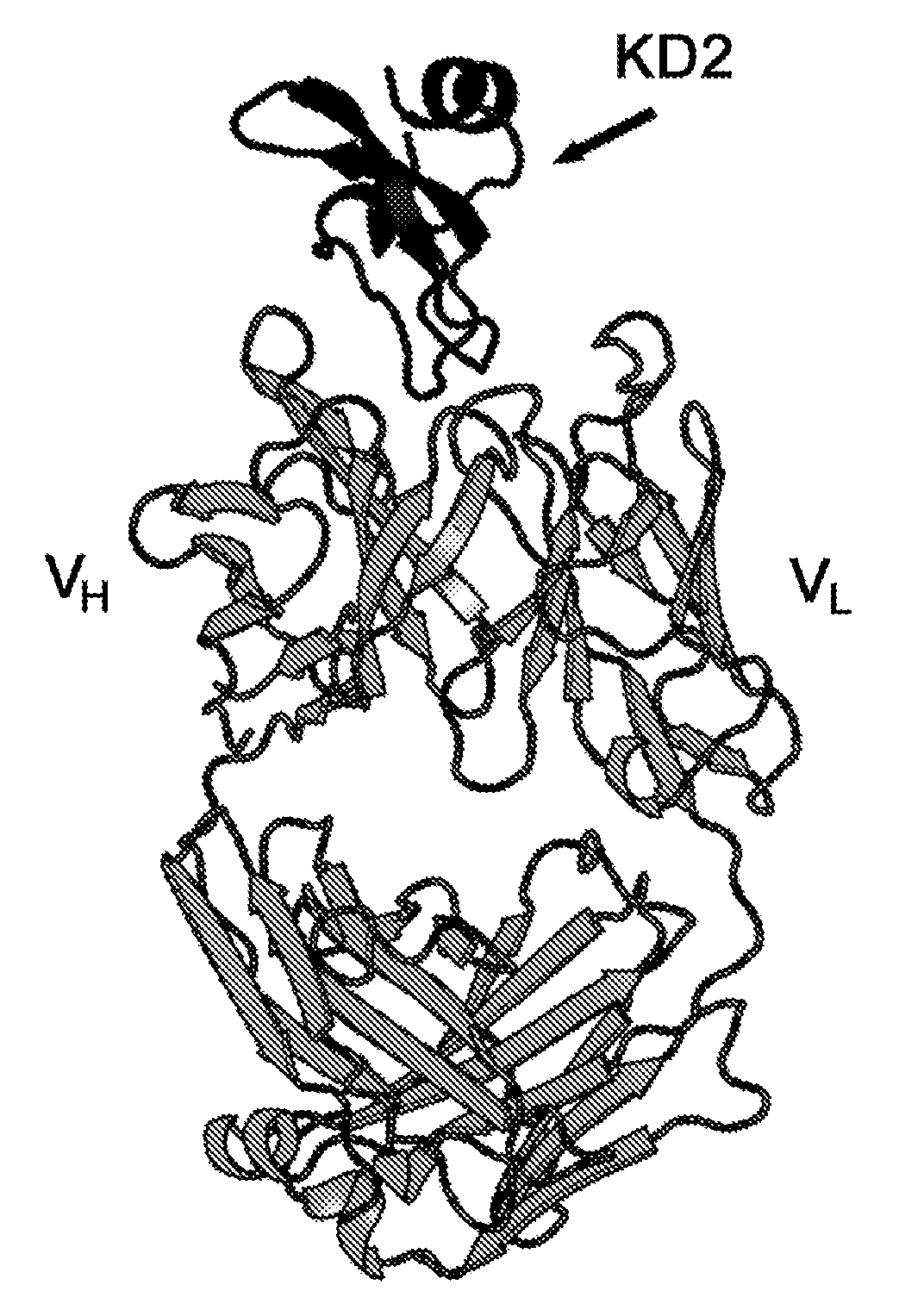
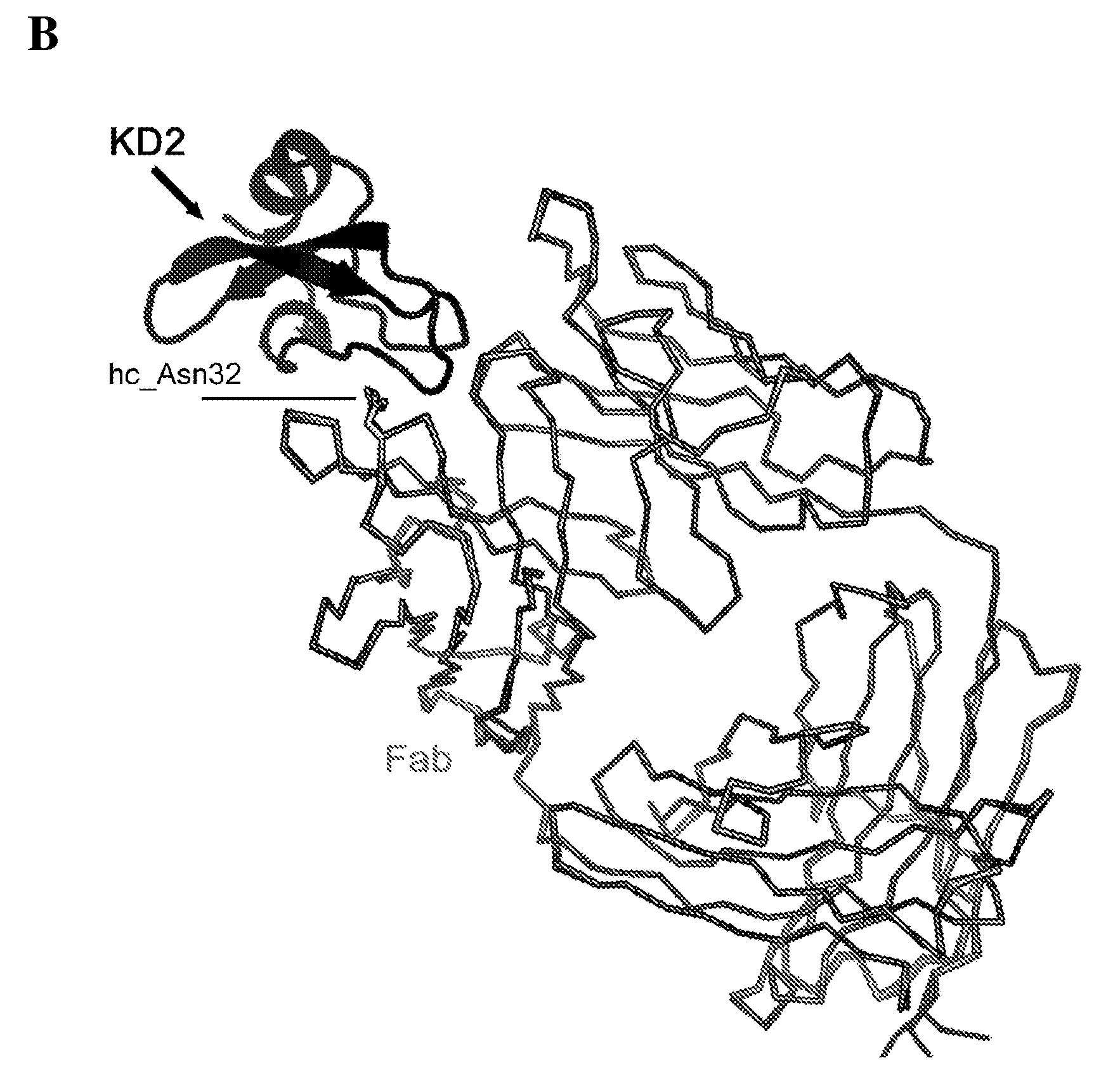
[0130] Example 3. Crystallization and X-ray structure determination of the TFPI-Fab A complex
[0131] crystallization
[0132] Co-crystals of TFPI Kunitz domain 2 and monoclonal antibody Fab A were grown at 20°C using the sessile drop method. The protein complex was concentrated to 9 mg / mL and crystallized by mixing equal volumes of protein solution and well solution (15% PEG8000, Tris HCl pH 7.5) as precipitant. Crystals appeared after a day.
[0133] Data Acquisition and Processing
[0134] Crystals were snap frozen in 30% glycerol in crystallization buffer in liquid nitrogen for cryoprotection. Data were collected at beamline BL14.1, BESSY synchrotron (Berlin) on a MAR CCD detector. Index the data and communicate with XDS
[0135]
[0136] Integrate, prepare for scale with POINTLESS (P.R. Evans, (2005) Acta Cryst. D62, 72-82) and scale with SCALA (P.R. Evans, (2005) Acta Cryst. D62, 72-82). The crystal diffracts up to 2.6 ? and has space group P2 1 2 1 2 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com