Primers, probe, kit and detection method for type II nucleic acid quantitative detection of herpes simplex virus
A herpes simplex virus, detection kit technology, applied in biochemical equipment and methods, microorganism-based methods, and microbial determination/inspection, etc., can solve the problems of false positives and high probe TM values
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Embodiment 1: the preparation of kit
[0070] Components of various reagents:
[0071] Lysate: (10mmMPH7.0) (sigma), silica (1‰), Triton X-100 (1‰), EDTA·2Na (10mM), guanidine isothiocyanate (2M);
[0072] detergent: (10mmMPH7.0), Triton X-100 (1‰), EDTA·2Na (1mM), guanidine isothiocyanate (2M);
[0073] Repeat wash solution: (10mmMPH7.0), Triton X-100 (1‰), EDTA·2Na (1mM)
[0074] HSVⅡPCR reaction tube:
[0075] Taq enzyme (2U), UNG enzyme (0.01U), EDTA·2Na (0.1mM), paraffin oil (25 microliters);
[0076] HSVⅡ reaction buffer: HSVⅡ upstream and downstream primers (10pM), HSVⅡ probe (5pM), (20Mmph9.0), KCl (50mM), MgCl2 (1.5mM), EDTA·2Na (0.1mM), dNtps (0.2mM);
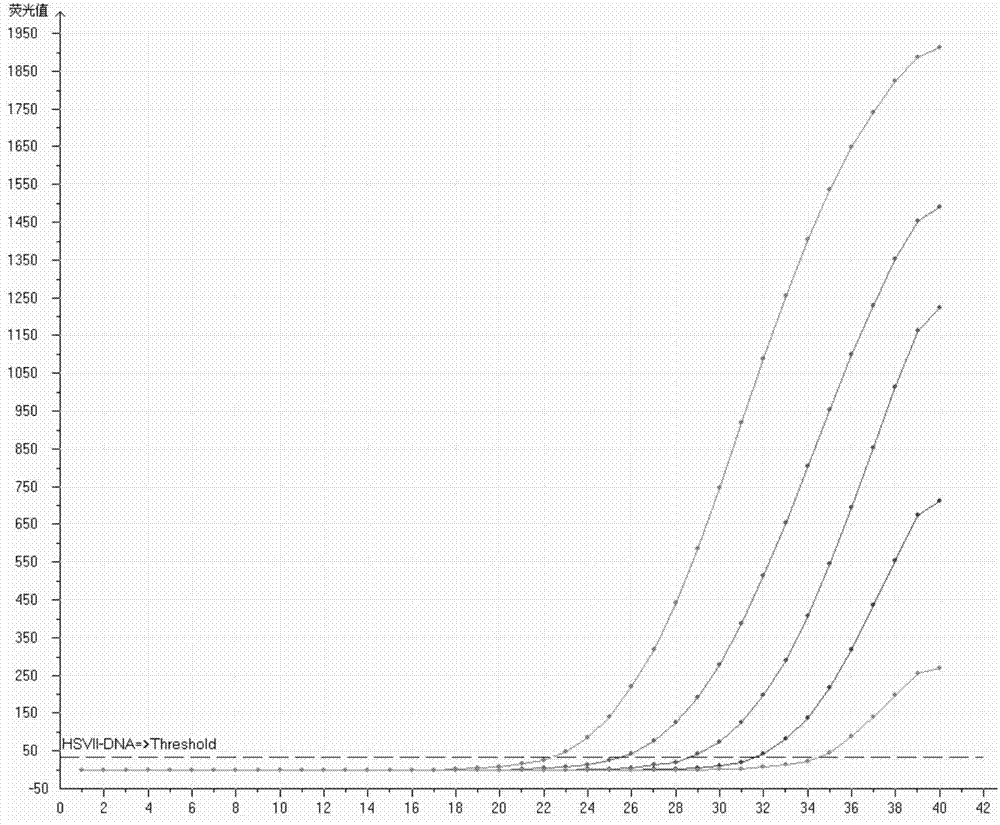
[0077] HSVⅡquantitative standards are shown in Table 2
[0078]
[0079] HSVⅡV negative control: normal saline;
[0080] HSVⅡ critical positive control: normal saline, virus-like particles;
[0081] HSVⅡ strong positive control: normal saline, virus-like particles.
[0082] The preparation me...
Embodiment 2
[0089] Example 2: Detection of samples using kits
[0090] (1) Sample
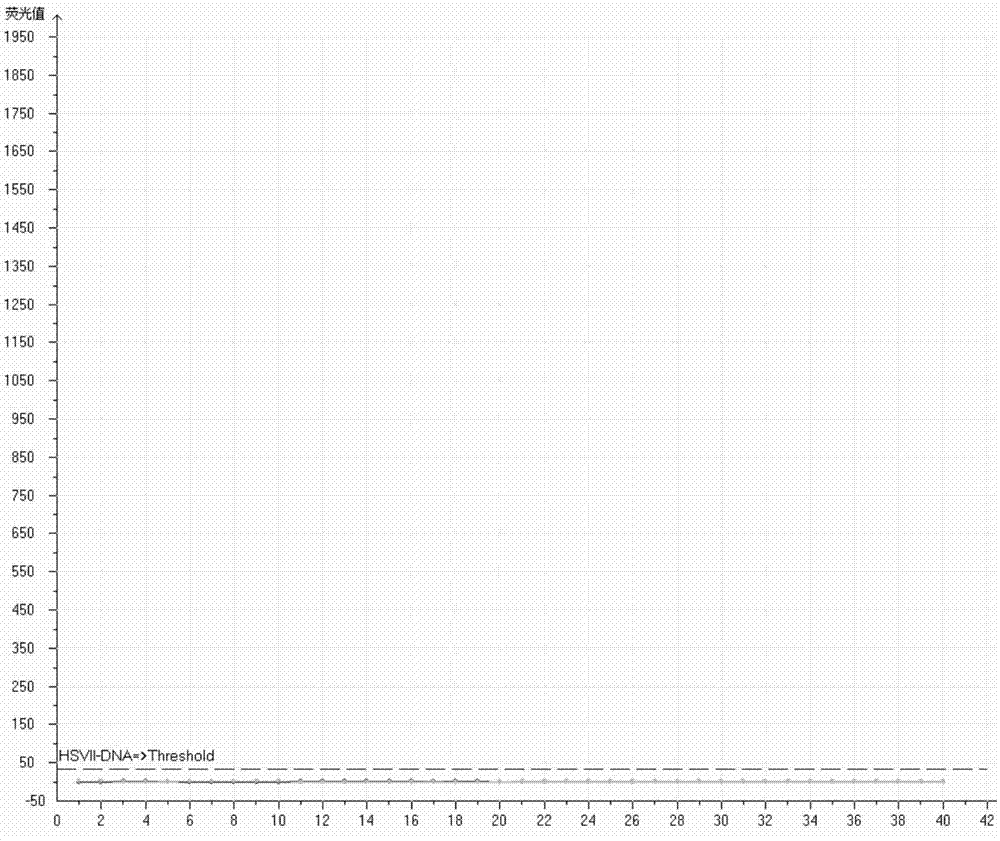
[0091] 1. 8 samples were negative
[0092] Clinical collection of HPV6 type secretion samples, HPV11 type vaginal discharge samples, HPV16 type vaginal discharge samples, HPV18 type vaginal discharge samples, Chlamydia trachomatis secretion samples, Neisseria gonorrhoeae secretion samples, Ureaplasma urealyticum secretion samples Samples, human cytomegalovirus urine samples, divided into centrifuge tubes at 165 μL / cartridge, labeled as N1, N2, N3, N4, N5, N6, N7, N8 in turn. (Xiamen Hospital of Traditional Chinese Medicine)
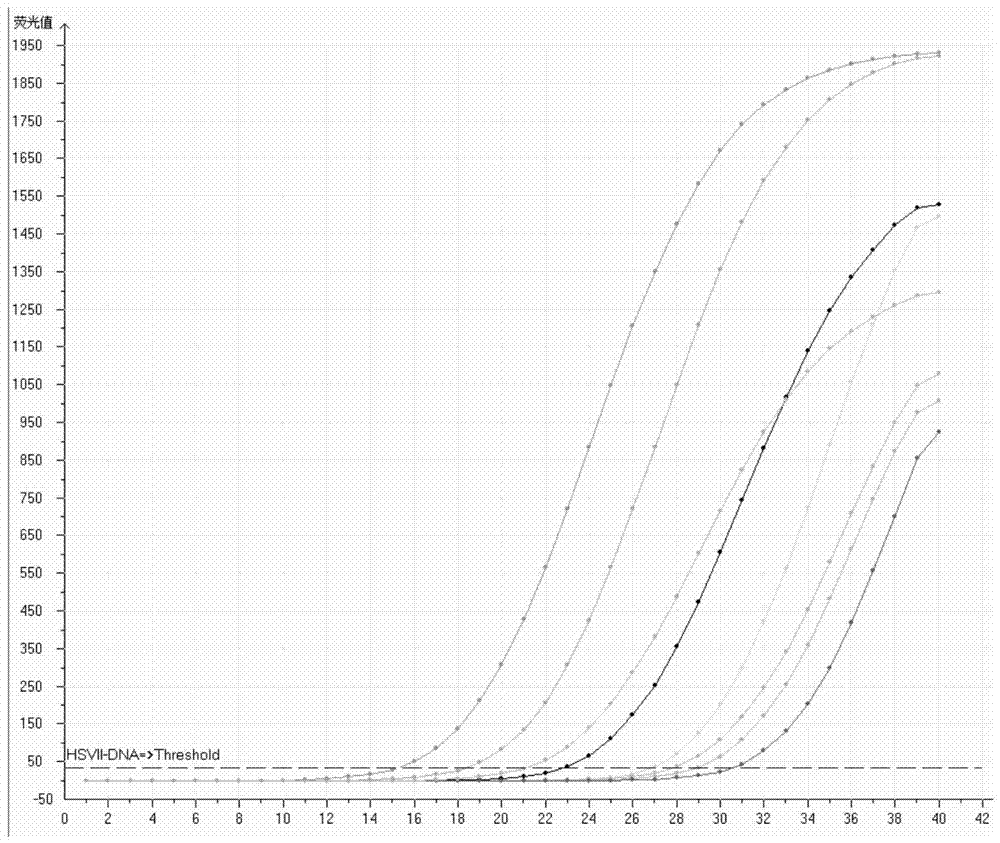
[0093] 2. 8 samples to be tested are positive (clinical collection of herpes simplex virus type II secretion samples, the concentration is P11.0×10 7 copies / mL, P21.0×10 6 copies / mL, P31.0×10 5 copies / mL, P41.0×10 5 copies / mL, P51.0×10 4 copies / mL, P61.0×10 4 copies / mL, p71.0×10 3 copies / mL), p81.0×10 3 copies / mL). (Xiamen Hospital of Traditional Chinese Medicine)
[0094]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com