Electrocatalyst with mixed precious metal and perovskite oxides
A perovskite oxide, perovskite technology, applied in circuits, electrical components, battery electrodes, etc., can solve the problems of high cost, low catalytic activity of oxygen reduction reaction, poor catalytic oxygen evolution reaction activity, etc. The effect of precious metal consumption, cost reduction, and simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
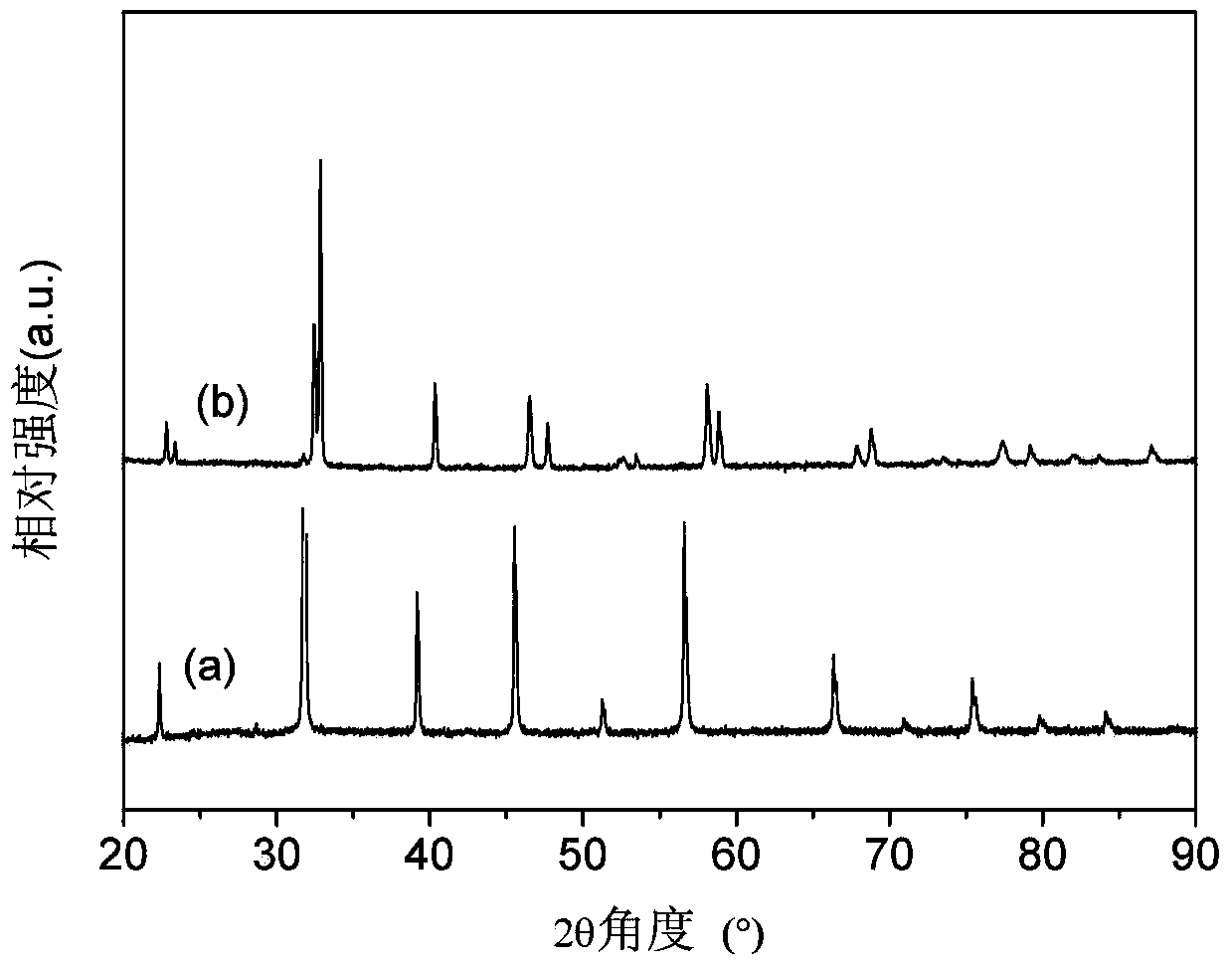
[0042] Weigh 4mg of Pt / C (precious metal mass fraction accounts for 40%, conductive carbon black accounts for 60%, the following examples 2-embodiment 10 are all like this) and 16mg BSCF / C (perovskite oxide mass fraction accounts for 40%) , conductive carbon black accounted for 60%, the following examples 2-embodiment 10 are all the same) according to the mass ratio of 1:4, mixed and dispersed in 1mL of ethanol, and then added Nafion (mass fraction 5%) 0.1mL, ultrasonic Shake for 1 h to make the mixture uniform to obtain a slurry for preparing the catalyst. The electrode preparation and electrode testing process are the same as those in Comparative Example 1.
[0043] figure 2 (a) is the SEM image of the mixed Pt and BSCF, and the results show that the nanoscale Pt is relatively uniformly distributed on the surface of the BSCF.
Embodiment 2
[0045] Weigh 10mg of Pt / C and 10mg of BSCF / C according to the mass ratio of 1:1, mix and disperse them in 1mL of ethanol, then add 0.1mL of Nafion (mass fraction 5%) to it, and ultrasonically oscillate for 1h to mix evenly to obtain the prepared catalyst. slurry. The electrode preparation and electrode testing process are the same as in Example 1.
Embodiment 3
[0047] Weigh 16mg of Pt / C and 4mg of BSCF / C according to the mass ratio of 4:1, mix and disperse in 1mL of ethanol, then add 0.1mL of Nafion (mass fraction 5%) to it, and ultrasonically oscillate for 1h to make the mixture uniform to obtain the prepared catalyst. slurry. The electrode preparation and electrode testing process are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com