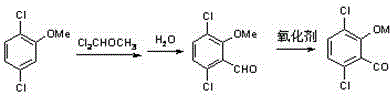
A kind of synthetic method of 3,6-dichloro-2-methoxybenzoic acid
The technology of a methoxybenzoic acid and a synthesis method is applied in the synthesis field of 3,6-dichloro-2-methoxybenzoic acid, and can solve the problems of high difficulty in industrialization, large discharge of three wastes, long reaction steps and the like, Achieve the effects of good product quality, reduced waste water, and short steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] 1) In a 500ml four-neck flask, dissolve 36g (98.5%) of 2,5-dichloroanisole in 100ml of dichloromethane, and add 0.2g of TiCl 4 , lower the temperature to 5°C, slowly add 100ml of methylene chloride solution containing 27.84g (99%) of dichloromethyl methyl ether dropwise under stirring conditions, add 200ml of water after the addition is completed, and adjust the pH to 3.0 with dilute sulfuric acid, at 25°C Stir for 4h. After standing still, the oil layer was separated to obtain 40.2 g of 2-methoxy-3,6-dichlorobenzaldehyde with a yield of 95%.
[0026] 2) Add 150ml of toluene solution of 21g (98%) 2-methoxy-3,6-dichlorobenzaldehyde into a 500ml four-necked flask, slowly add 24.9g of 30% NaClO solution under stirring condition, and control the temperature at 25°C , Stir the reaction for 5h. Toluene was distilled off under reduced pressure, dilute sulfuric acid was added to the aqueous solution to adjust the pH to 1.0, the temperature was lowered, and after filtration an...
Embodiment 2
[0028] 1) In a 500ml four-neck flask, dissolve 37g (97.0%) of 2,5-dichloroanisole in 100ml of dichloroethane, and add 0.4g of TiCl 4 , lower the temperature to 10°C, maintain the temperature, and slowly add 100ml of dichloroethane solution containing 21.0g (99%) of dichloromethyl methyl ether dropwise under stirring conditions, add 300ml of water after the addition is completed, and adjust the pH to 1.5 with sulfuric acid , Stir at 20°C for 3h. After standing still, the oil layer was separated to obtain 39.2 g of 2-methoxy-3,6-dichlorobenzaldehyde, with a yield of 93.7%.
[0029] 2) Add 21g (98%) 2-methoxy-3,6-dichlorobenzaldehyde in 150ml of dichloroethane solution into a 500ml four-necked flask, slowly add 60g of 20% NaBrO solution under stirring condition, and control the temperature 20 ° C, stirring the reaction for 3h. Dichloroethane was distilled off under reduced pressure, and an appropriate amount of dilute sulfuric acid was added to the remaining aqueous solution to...
Embodiment 3
[0031] 1) In a 500ml four-neck flask, dissolve 37g (97.0%) of 2,5-dichloroanisole in 100ml of toluene, and add 0.3g of TiCl 4 , cool the cold brine to 0°C, slowly add 100ml of toluene solution dissolved with 26g of dichloromethyl methyl ether dropwise under stirring conditions, add 100ml of water after the dropwise addition, adjust the pH to 2.0 with sulfuric acid, and stir at 15°C for 6h. After standing still, the oil layer was separated to obtain 39.8 g of 2-methoxy-3,6-dichlorobenzaldehyde, with a yield of 95.14%.
[0032] 2) Add 150ml of dichloroethane solution of 21g (98%) 2-methoxy-3,6-dichlorobenzaldehyde into a 500ml four-neck flask, slowly add 15g of 30% hydrogen peroxide solution under stirring condition, and control the temperature 15°C, stirred and reacted for 8h. The solvent was distilled off under reduced pressure, and an appropriate amount of dilute sulfuric acid was added to the remaining aqueous solution to adjust the pH to 2.0. After filtration and drying, 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com