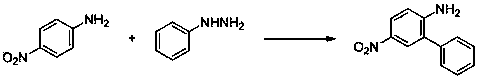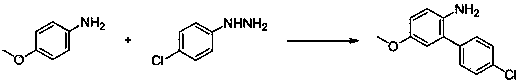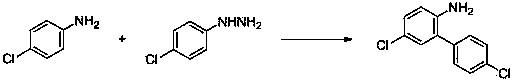Method for preparing 2-amino biphenyl derivative
A technology of aminobiphenyl and aniline derivatives, which is applied in the preparation of amino hydroxyl compounds, organic compounds, amino compounds from amines, etc., can solve the problems of low resource utilization, unfavorable environment, high production cost, etc., and achieve various , Raw materials are easy to obtain, and the ratio of raw materials is reasonable
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment one: the synthesis of 2-aminobiphenyl
[0041] With aniline and phenylhydrazine as raw materials, the reaction formula is as follows:
[0042]
[0043] (1) Add 0.47 g (5 mmol) of aniline, 0.108 g (1 mmol) of phenylhydrazine, 0.057 g (0.1 mmol) of iron phthalocyanine and 10 ml of methanol into the reaction flask, and react at 20°C;
[0044] (2) TLC tracking reaction until complete completion;
[0045] (3) The crude product obtained after the reaction was separated by column chromatography (petroleum ether: ethyl acetate = 20:1) to obtain the target product (yield 72%).
[0046] 1 H NMR (400 MHz, CDCl 3 ): δ 7.40-7.49 (m, 4H), 7.30-7.38 (m, 1H), 7.10-7.20 (m, 2H), 6.76-6.89 (m, 2H), 4.08 (br, s, 2H); 13 C NMR (75 MHz, CDCl 3 ): δ 143.0, 139.4, 130.5, 129.1, 128.8, 128.5, 128.0, 127.2, 119.0, 115.9.
Embodiment 2
[0047] Example 2: Synthesis of 2-amino-5-fluorobiphenyl
[0048] With 4-fluoroaniline and phenylhydrazine as raw materials, the reaction formula is as follows:
[0049]
[0050] (1) Add 1.11 g (10 mmol) of 4-fluoroaniline, 0.108 g (1 mmol) of phenylhydrazine, 0.057 g (0.1 mmol) of iron phthalocyanine and 10 ml of ethanol to the reaction flask, and react at 70°C;
[0051] (2) TLC followed the reaction until it was completely completed; the crude product obtained after the reaction was separated by column chromatography (petroleum ether: ethyl acetate = 20:1) to obtain the target product (yield 71%).
[0052] 1 H NMR (400 MHz, CDCl 3 ): δ 7.34-7.40 (m, 4H), 7.25-7.33 (m, 1H), 6.78-6.87 (m, 2H), 6.73 (dd, J = 5.6, 9.4 Hz, 1H); 13 C NMR (100 MHz, CDCl 3 ): δ 157.0 (d, J = 237.9 Hz), 138.2, 137.7, 129.0, 128.9, 127.8, 117.6 (d, J = 7.8 Hz), 116.8 (d,J = 22.5 Hz), 114.9 (d, J = 22.3 Hz).
Embodiment 3
[0053] Embodiment three: the synthesis of 2-amino-5-chlorobiphenyl
[0054] With 4-chloroaniline, phenylhydrazine as raw materials, its reaction formula is as follows:
[0055]
[0056] Add 0.89 g (7 mmol) of 4-chloroaniline, 0.108 g (1 mmol) of phenylhydrazine, 0.057 g (0.1 mmol) of iron phthalocyanine (0.1 mmol) and 10 ml of acetonitrile into the reaction flask, and react at 80 ° C; follow the reaction by TLC until it is completely completed; The crude product obtained after the reaction was separated by column chromatography (petroleum ether: ethyl acetate = 20:1) to obtain the target product (yield 69%).
[0057] 1 H NMR (400 MHz, CDCl 3 ): δ 7.40-7.47 (m, 4H), 7.33-7.39 (m, 1H), 7.08-7.14 (m, 2H), 6.74 (d, J = 8.9 Hz, 1H), 4.20 (br, s, 2H); 13 C NMR (75 MHz, CDCl 3 ): δ 141.1, 137.2, 128.9, 127.9, 127.8, 127.1, 126.6, 122.1, 115.6.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com