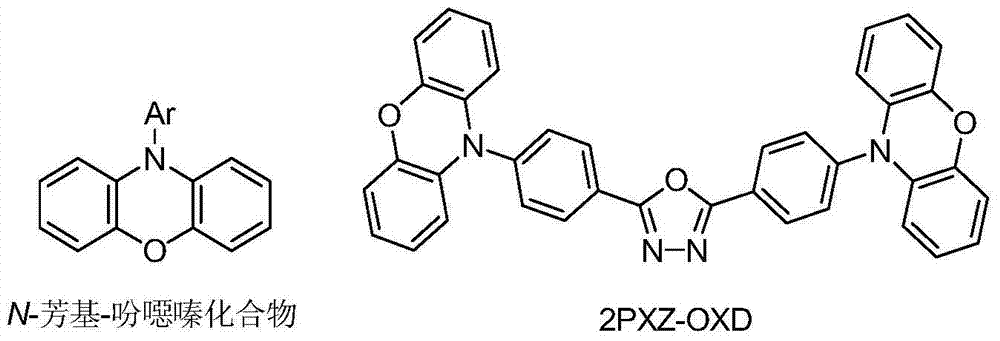
Method for synthesizing N-aryl-phenoxazine compounds
A technique of -aryl-, compound, applied in the field of organic synthetic chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
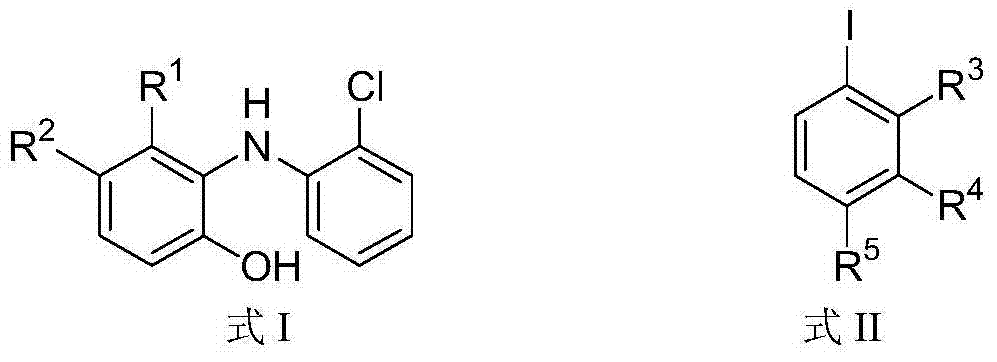
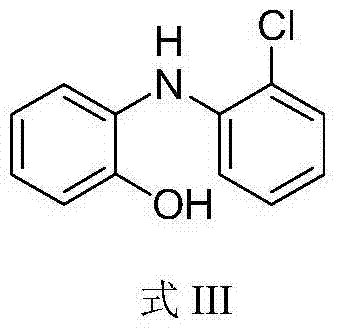
[0047] The preparation of N-phenyl-phenoxazine (formula I-a): 2-[N-(2-chlorophenyl)-amino]-phenol (formula III, 1.0mmol), CuI (0.05mmol) and Cs 2 CO 3 (3.0 mmol) and n-PrCN (2 mL) were added to a Schlenk tube. Firstly, the air was removed through three repetitions of evacuation and nitrogen injection, and then iodobenzene (1.1 mmol) was injected with a needle. After the resulting suspension was heated to reflux for 24 hours, the solids were filtered off. After removing the solvent on a rotary evaporator, the residue was purified by column chromatography [silica gel, EtOAc:petroleum ether (60-90°C)=1:99 (volume ratio)] to obtain N-phenyl-phenone in a yield of 98%. Oxazine, the structural formula is as follows:
[0048]
[0049] mp140~141℃[138–139℃; references: Gilman, H.; Moore, L.O.J.Am.Chem.Soc.1957,79,3485-3487.];
[0050] 13 C NMR (CDCl 3 )δ 143.9(2C), 138.9, 134.4, 131.0(2C), 130.8(2C), 128.4(2C), 123.2(2C), 121.2(2C), 115.4(2C), 113.2(2C).
[0051] Indicates tha...
Embodiment 2
[0053] The preparation of N-(2-methylphenyl)-phenoxazine (formula I-b): using the same conditions as in Example 1, only iodobenzene is replaced by o-iodotoluene to obtain N- (2-Methylphenyl)-phenoxazine, the structural formula is as follows:
[0054]
[0055] White crystal, mp171~173℃;
[0056] IR(KBr)ν1633, 1485, 1334, 1269, 728cm -1 ;
[0057] 13 C NMR (CDCl 3 )δ143.9(2C),138.9,136.8,133.4,132.2(2C),131.0,128.9(2C),128.6(2C),123.4(2C),121.1,115.4,112.6(2C),17.6;
[0058] HRMS(ESI-TOF)(m / z): Calculated value: [M] + 273.1148; Found: 273.1150.
[0059] Indicates that the obtained compound is correct.
Embodiment 3
[0061] The preparation of N-(3-methylphenyl)-phenoxazine (formula I-c): using the same conditions as in Example 1, only iodobenzene is replaced by m-iodotoluene to obtain N- (3-Methylphenyl)-phenoxazine, the structural formula is as follows:
[0062]
[0063] White crystal, mp123~125℃;
[0064] IR(KBr)ν1636, 1485, 1335, 1269, 732cm -1 ;
[0065] 13 C NMR (CDCl 3 )δ143.9(2C),141.2,138.8,134.4(2C),131.1,130.7,129.2,127.6,123.1(2C),121.1(2C),115.3(2C),113.2(2C),21.3;
[0066] HRMS(ESI-TOF)(m / z): Calculated value: [M] + 273.1148; Found: 273.1145.
[0067] Indicates that the obtained compound is correct.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com