Chinese medicinal composition for treating diabetic nephropathy and preparation method thereof
A technology for diabetic nephropathy and a composition is applied in the field of traditional Chinese medicine compositions for the treatment of diabetic nephropathy and the field of preparation thereof, which can solve problems such as incomplete elucidation of the pathogenesis, and achieve the effects of improving renal function, easy preparation and molding, and reducing urinary protein.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0106] (1) Weigh Chinese herbal medicines according to the following weight proportions, 20 parts of Astragalus membranaceus, 10 parts of Rosa laevigata, 10 parts of Rhizoma Chuanxiong, 15 parts of hollyhock flower, 5 parts of rhubarb, 15 parts of Dahuacao, and 10 parts of Pueraria lobata.
[0107] (2) Add astragalus, kudzu root, chuanxiong, and hollyhock flowers to 12 times the weight of 63% ethanol for reflux extraction twice, each time for 150 minutes, and collect the extract.
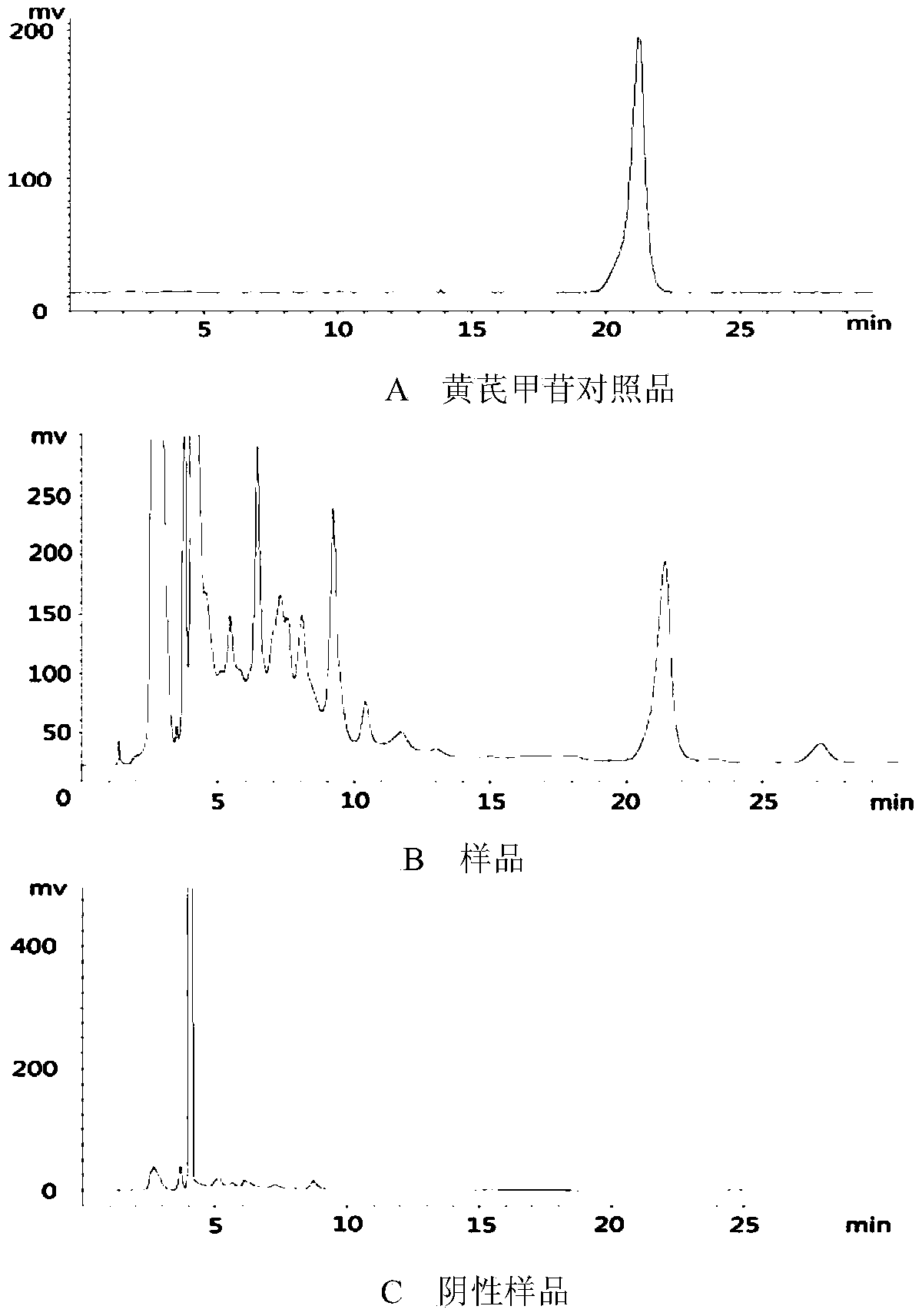
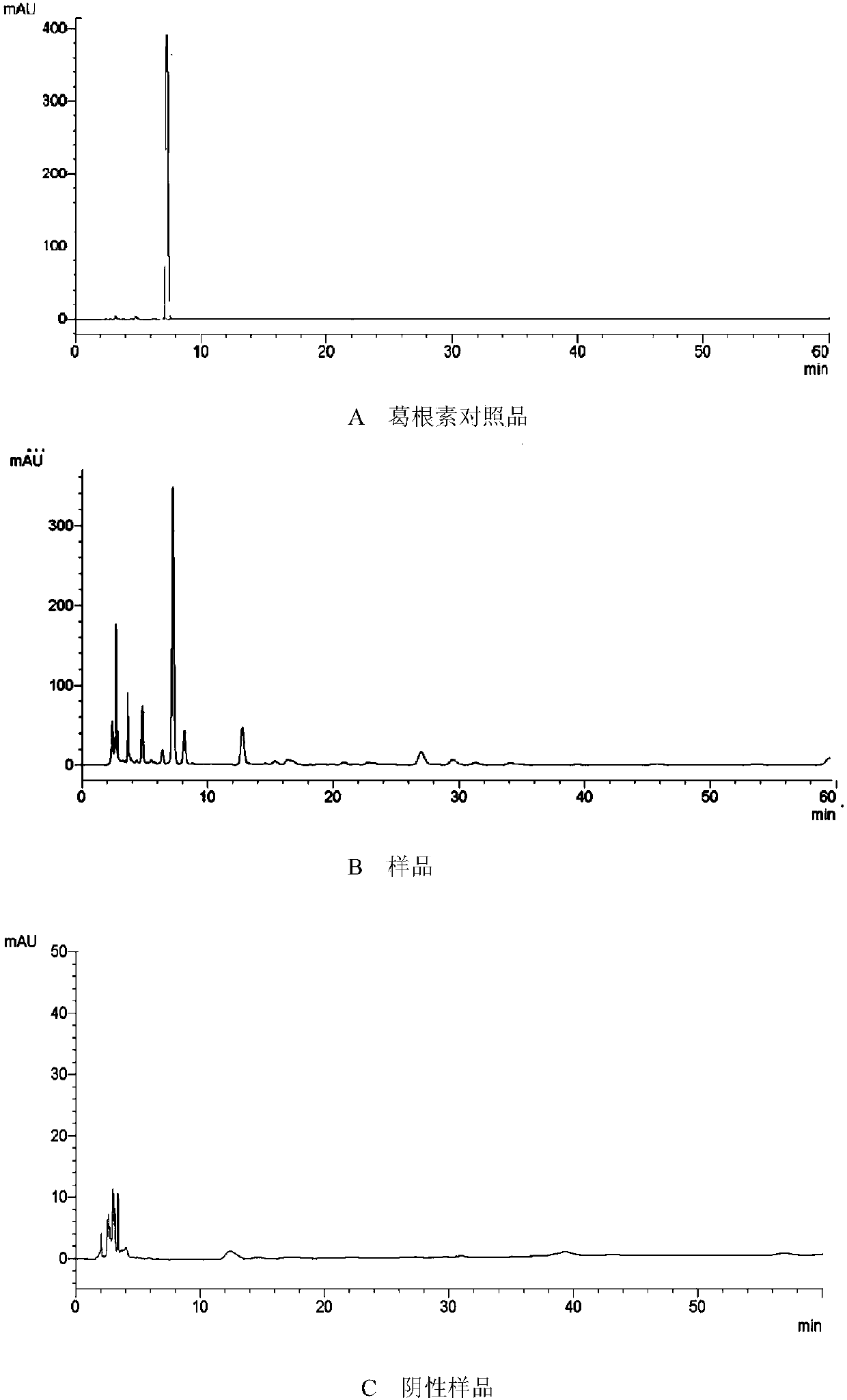
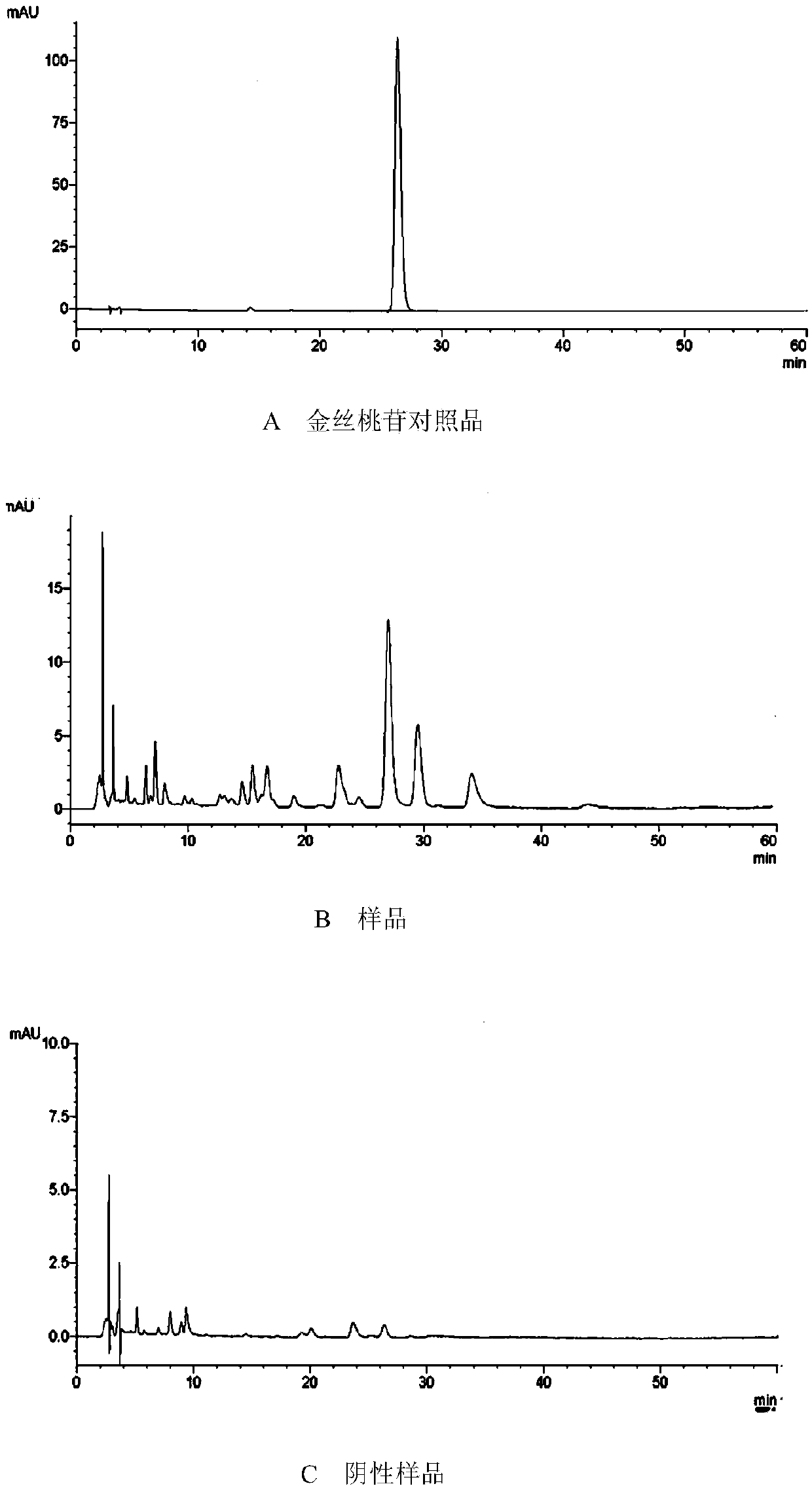
[0108] Determination of the contents of astragaloside IV, hyperoside and puerarin by high performance liquid chromatography. Determination of the content of astragaloside IV, detector: evaporative light scattering detector; the chromatographic column of high performance liquid chromatography is C 18 ; Mobile phase: acetonitrile-water (32:68); Mobile phase flow rate: 1.0 mL / min; Drift tube temperature: 101° C.; Carrier gas flow rate: 2.7 L / mL; Determination of hyperin and puerarin content, detector:...
Embodiment 2
[0117] The Chinese medicinal materials were weighed according to the following weight proportions, 15 parts of Astragalus membranaceus, 13 parts of Fructus Rosa, 7 parts of Rhizoma Chuanxiong, 15 parts of Sunflower of hollyhock, 3 parts of rhubarb, 15 parts of Rhizoma chinensis, and 13 parts of Pueraria lobata.
[0118]Add Radix Astragali, Pueraria Radix Puerariae, Ligusticum Chuanxiong, and hollyhock flowers by weight to 14 times of 50% ethanol to reflux and extract once, each time for 180 minutes, collect the extract, and use the same method as in Example 1 to measure the content of astragaloside IV as 1.3074mg / g, the content of puerarin is 39.6242mg / g, the content of hyperin is 7.1072mg / g, be concentrated to the clear cream that relative density is 1.1; Water reflux extraction 2 times, each 1 hour, collect extract, adopt the method identical with embodiment 1 to measure the content of rhein to be 2.7084mg / g, be concentrated to the clear cream that relative density is 1.1; ...
Embodiment 3
[0120] The Chinese medicinal materials were weighed according to the following weight proportions, 20 parts of Astragalus membranaceus, 7 parts of Fructus Rosa, 10 parts of Rhizoma Chuanxiong, 10 parts of hollyhock flower, 3 parts of rhubarb, 10 parts of Rhizoma chinensis, and 7 parts of Pueraria lobata.
[0121] Astragalus, Radix Puerariae, Rhizoma Chuanxiong, and hollyhock flowers were added by weight with 8 times of 70% ethanol to reflux and extract 4 times, each time for 30 minutes, the extract was collected, and the content of astragaloside IV was measured in the same way as in Example 1 to be 1.2099mg / g, the content of puerarin is 40.2475mg / g, the content of hyperin is 7.0431mg / g, be concentrated to the clear paste that relative density is 1.1; Add rhubarb, Rosa laevigata, Scutellaria chinensis 8 times by weight Water reflux extracts 4 times, each 0.5 hour, collects extract, adopts the method identical with embodiment 1 to measure the content of rhein to be 2.5489mg / g, b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com