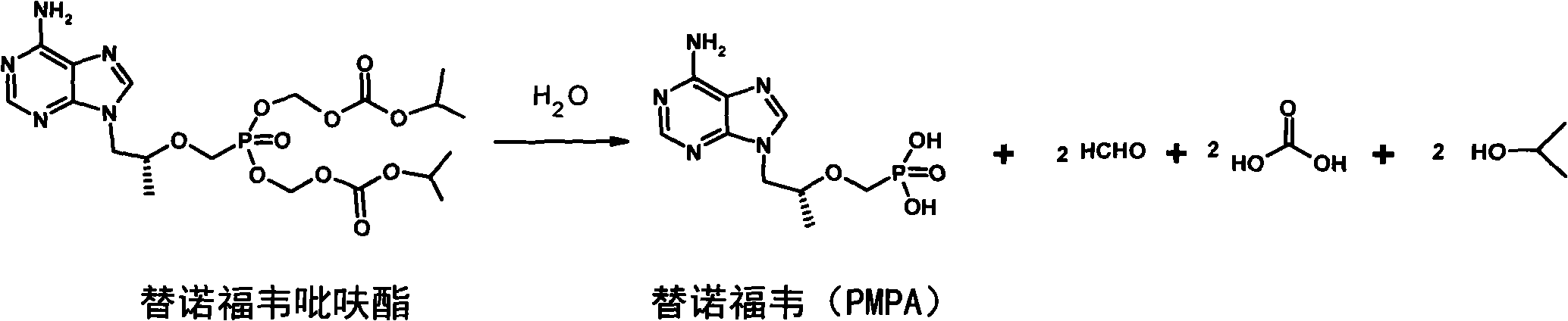
Hepatitis treatment medicament
A drug, hepatitis technology, applied in the field of hepatitis treatment drugs, can solve problems such as slow onset
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
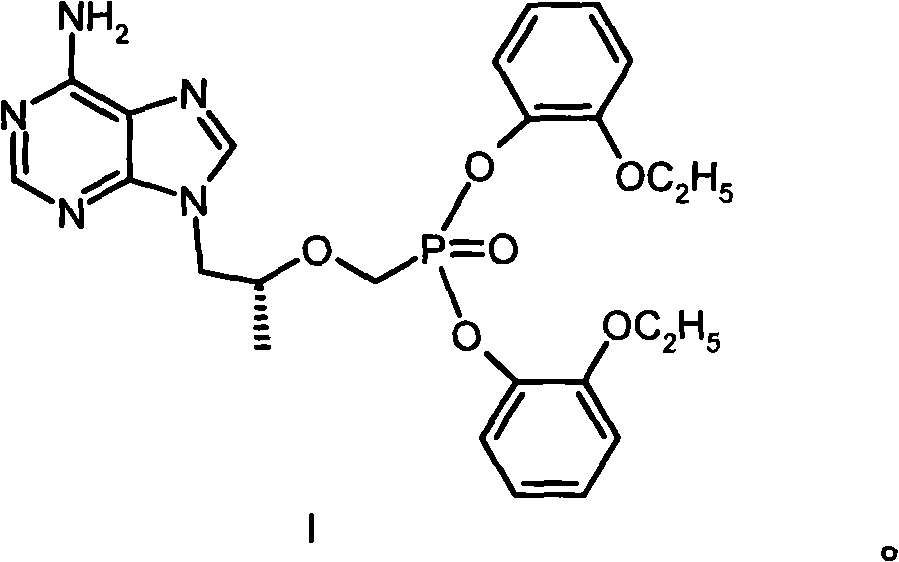
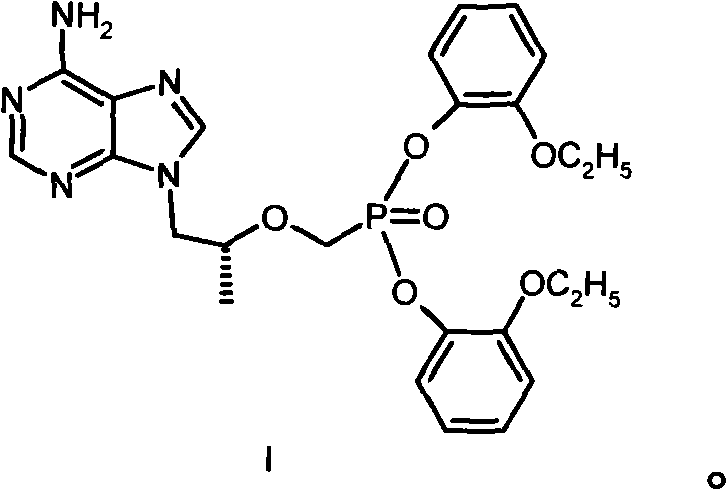
[0027] Example 1 [[(R)-2-(6-amino-purin-9-yl)-1-methyl-ethoxy-]methyl]phosphonic acid bis(2-ethoxy)phenyl ester (I ) preparation
[0028] 144g (0.50mol) [[(R)-2-(6-amino-purin-9-yl)-1-methyl-ethoxy-]methyl]phosphonic acid, 276g (2.00mmol) o-ethoxy Base phenol and 390g of 1-methyl-2-pyrrolidone were heated to 85°C, then 63g of triethylamine was added, 309g (1.50mmol) of DCC was added, and heated and stirred at 100°C for 16 hours. After cooling, the solid was filtered off; the filtrate was concentrated under reduced pressure, separated by a silica gel (200-300 mesh) column, eluted with a mixed solvent of dichloromethane: methanol (20:1), and the required components were collected and evaporated to dryness under reduced pressure. The residue was recrystallized from ethyl acetate to obtain 35 g of I with a melting point of 110-112°C. Elemental Analysis C 25 h 27 N 5 o 6 Calculated P: C 56.92%, H 5.73%, N 13.28%, P 5.87%. Found values: C 56.72%, H 5.71%, N 13.18%, P 5.88%. ...
Embodiment 2
[0029] Example 2 [[(R)-2-(6-amino-purin-9-yl)-1-methyl-ethoxy-]methyl]phosphonic acid bis(2-ethoxy)phenyl ester salt Preparation of acid salt (I·HCl)
[0030] Dissolve 13g of I in 50ml of acetone, add diethyl ether solution of hydrogen chloride dropwise until the pH is 2-3, reflux for 10min, cool down naturally, precipitate a solid, and dry to obtain 13.3g of I·HCl with a melting point of 178-186°C. Elemental Analysis C 25 h 27 N 5 o 6 Calculated value of P·HCl: C 53.24%, H 5.54%, Cl 6.29%, N 12.42%, P 5.49%; measured value (%): C 53.31%, H 5.58%, Cl 6.19%, N 12.41%, P 5.40 %.
Embodiment 3
[0031] Embodiment 3 stability test
[0032] Determine the content of the sample according to high performance liquid chromatography (Chinese Pharmacopoeia 2000 edition two appendix V D).
[0033] Chromatographic conditions and system suitability test: amino-bonded silica gel was used as filler; acetonitrile-0.05mol / L potassium dihydrogen phosphate aqueous solution (22:78) was used as mobile phase, flow rate was 1.0ml / min, and detection wavelength was 260nm. The number of theoretical plates calculated by tenofovir disoproxil should not be less than 2000.
[0034]Determination method: Take about 25mg of the sample to be tested, weigh it accurately, put it in a 25ml measuring bottle, add mobile phase to dissolve and dilute to the mark, shake well, accurately draw 5ml, put it in a 25ml measuring bottle, add mobile phase to dilute to the mark, shake Uniform, as the test solution. Precisely measure 10 μl of the test solution, inject it into the liquid chromatograph, and record the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com