Preparation method of sulfur hexafluoride
A technology of sulfur hexafluoride and sulfur hexafluoride gas, applied in the direction of sulfur and halogen compounds, can solve the problems of energy consumption, increase purification, and many processes, and achieve the effect of saving purification process, increasing output and reducing pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
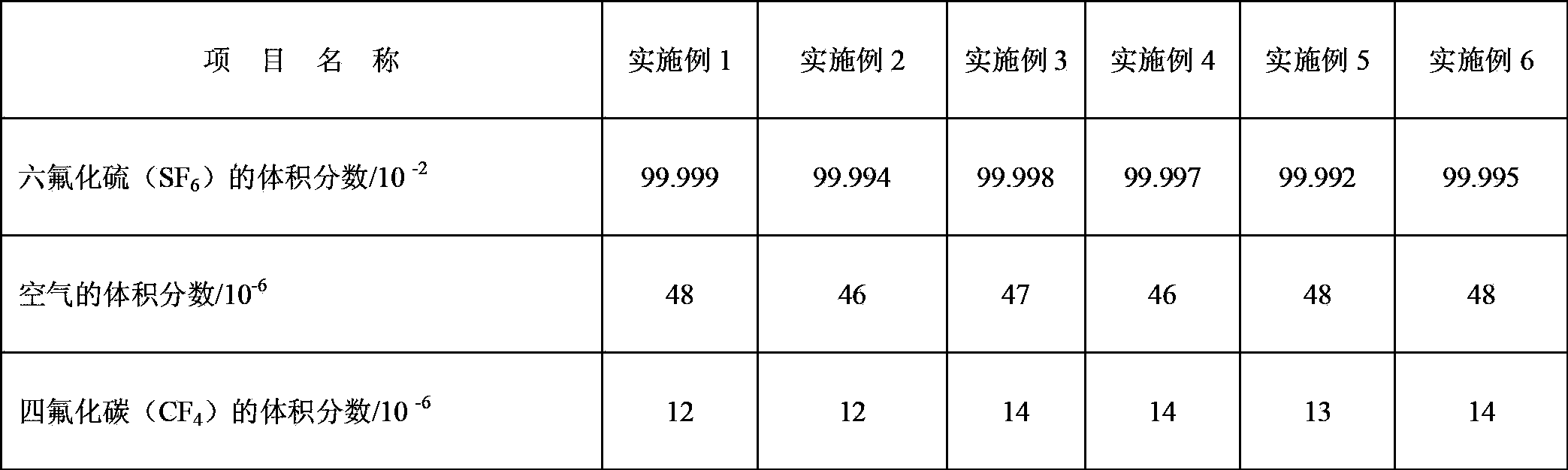
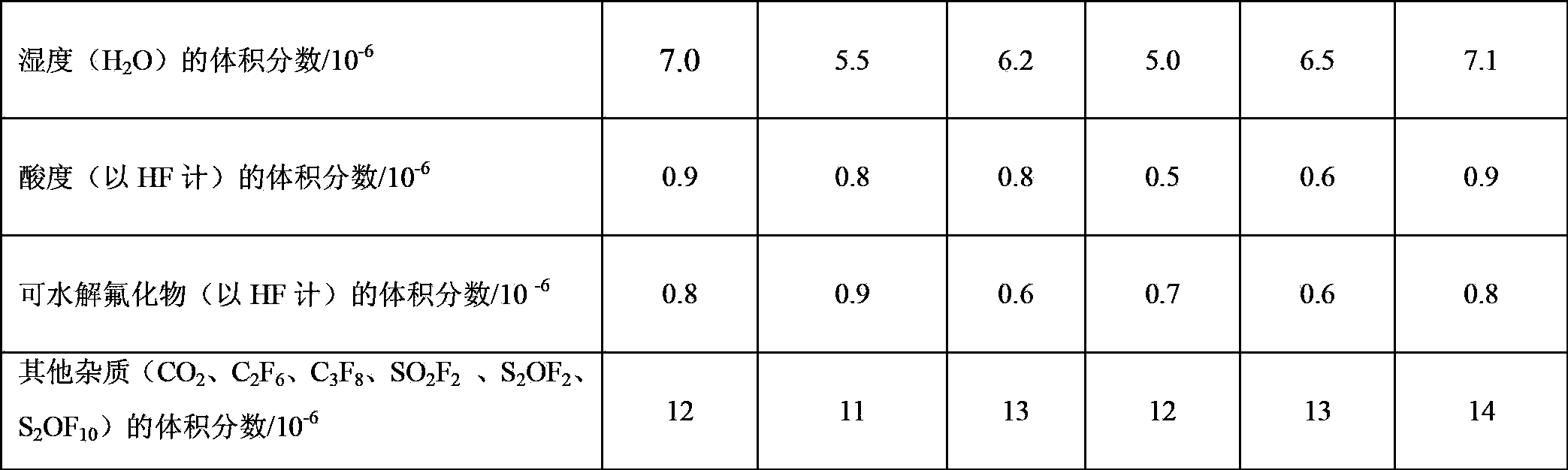
Examples
Embodiment 1
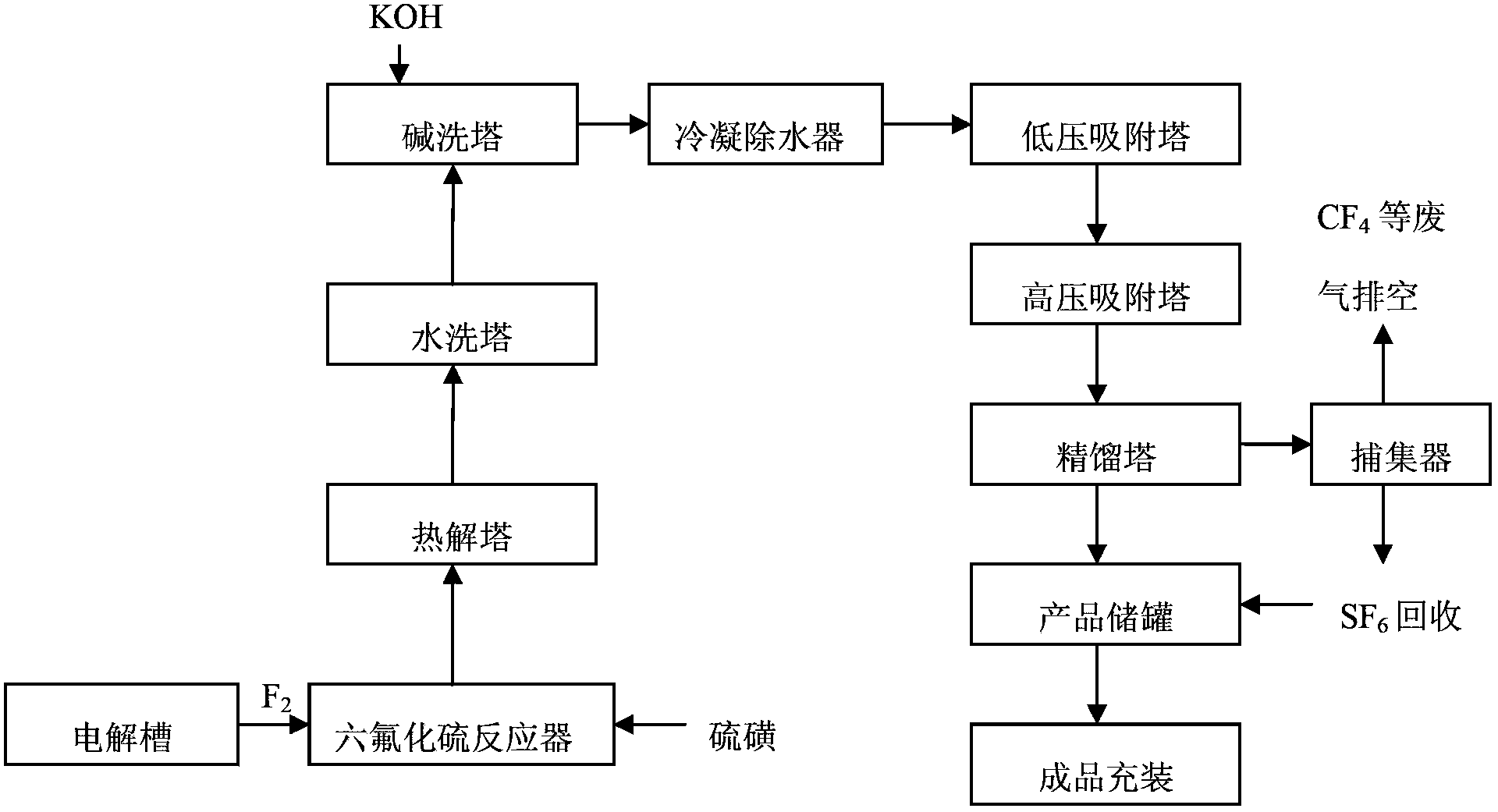
[0039] 1) Reaction
[0040] React fluorine gas and gaseous sulfur in a reactor to produce crude sulfur hexafluoride gas, wherein the fluorine gas is electrolyzed by a medium-temperature method using potassium bifluoride molten salt as the electrolyte and anhydrous hydrogen fluoride as the raw material The weight ratio of the fluorine gas to the gaseous sulfur is 1.05:1, the reaction temperature is 150°C, and the reaction pressure is -100Pa.
[0041] 2) Pyrolysis
[0042] Pass the generated crude sulfur hexafluoride gas into the pyrolysis tower, and pyrolyze to remove the S 2 f 10 , S 2 f 10 O poisonous gas. Wherein, the temperature of the pyrolysis tower is 380°C, and the pressure is -150Pa.
[0043] 3) washing
[0044] Pass the pyrolyzed crude sulfur hexafluoride gas into the water washing tower to hydrolyze the hydrolyzable impurities, and then pass the crude sulfur hexafluoride gas into the alkali washing tower to remove the acidity in the crude sulfur hexafluoride g...
Embodiment 2
[0054] 1) Reaction
[0055] The fluorine gas and the gaseous sulfur react in the reactor to generate crude sulfur hexafluoride gas. Wherein, the fluorine gas is produced by medium-temperature electrolysis with potassium bifluoride molten salt as the electrolyte and anhydrous hydrogen fluoride as the raw material, the weight ratio of the fluorine gas to the gaseous sulfur is 1.03:1, and the reaction temperature The temperature is 180°C, and the reaction pressure is -50Pa.
[0056] 2) Pyrolysis
[0057] Pass the generated crude sulfur hexafluoride gas into the pyrolysis tower to remove S 2 f 10 , S 2 f 10 O poisonous gas. Wherein, the temperature of the pyrolysis tower is 420°C, and the pressure is -200Pa.
[0058] 3) washing
[0059] Pass the pyrolyzed crude sulfur hexafluoride gas through the water washing tower to hydrolyze the hydrolyzable impurities, and then pass it into the alkali washing tower to neutralize and remove the acidic impurities in the crude sulfur hex...
Embodiment 3
[0069] 1) Reaction
[0070] Fluorine gas is reacted with gaseous sulfur in a reactor to produce crude sulfur hexafluoride gas. Wherein, the fluorine gas is produced by medium-temperature electrolysis with potassium bifluoride molten salt as the electrolyte and anhydrous hydrogen fluoride as the raw material. The weight ratio of the fluorine gas to the gaseous sulfur is 1.04:1, and the reaction temperature The temperature is 160°C, and the reaction pressure is -60Pa.
[0071] 2) Pyrolysis
[0072] Pass the generated crude sulfur hexafluoride gas into the pyrolysis tower to remove S 2 f 10 , S 2 f 10 O poisonous gas. Wherein, the temperature of the pyrolysis tower is 390°C, and the pressure is -160Pa.
[0073] 3) washing
[0074] Pass the pyrolyzed crude sulfur hexafluoride gas into the water washing tower to hydrolyze the hydrolyzable impurities, and then pass it into the alkali washing tower to neutralize and remove the acidic impurities in the crude sulfur hexafluorid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com