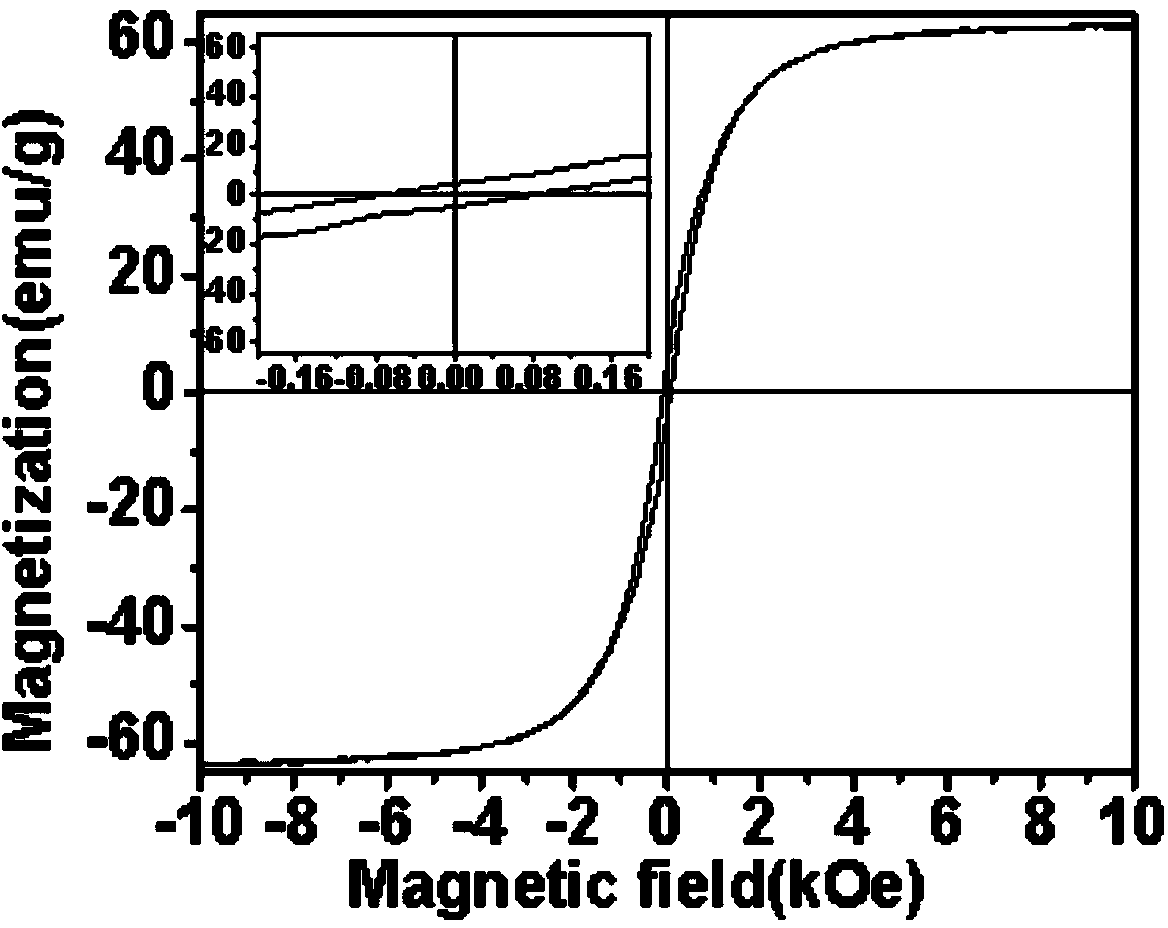
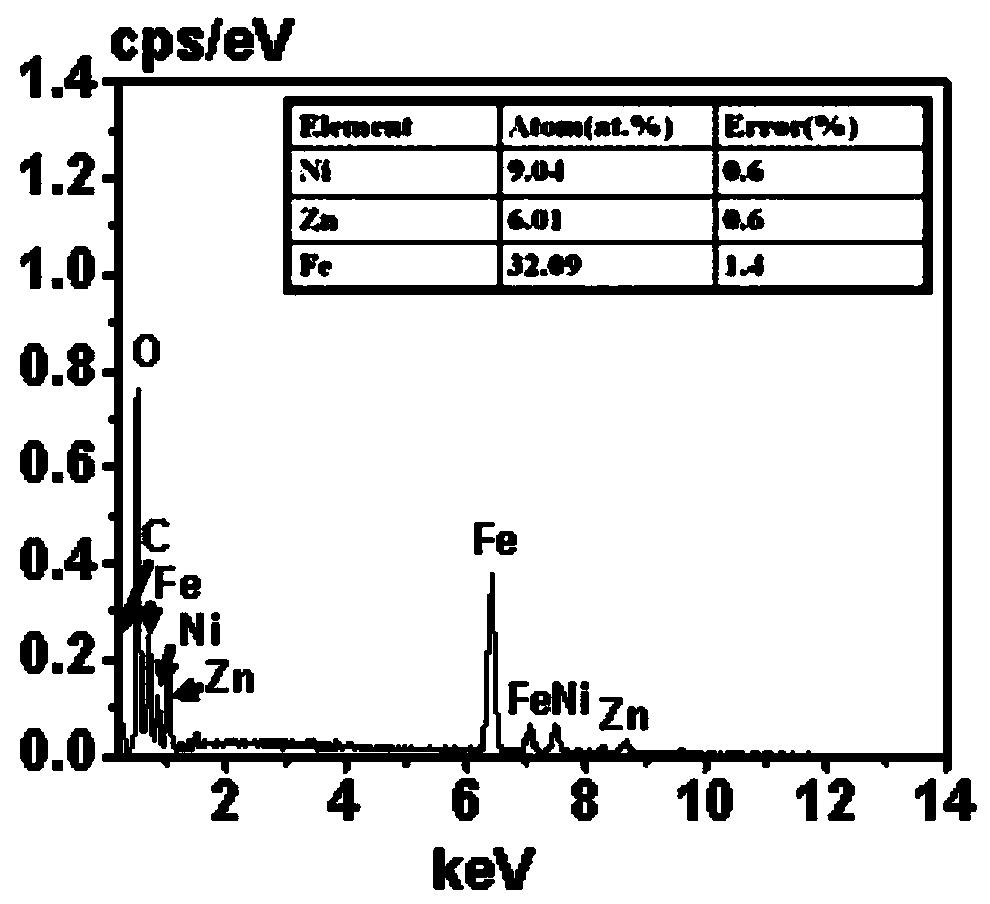
Preparation method of nickel-zinc ferrite/polyaniline composite material
A technology of nickel-zinc ferrite and composite materials, applied in the field of electromagnetic wave absorbing materials, can solve the problems of the influence of wave absorbing performance, less PANI composite materials, etc., and achieve the effects of enhancing wave absorbing performance, low preparation cost, and improved stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The molar ratio of aniline monomer to nickel zinc ferrite n(Ani):n(Ni 0.6 Zn 0.4 Fe 2 o 4 )=1:1 Ni 0.6 Zn 0.4 Fe 2 o 4 / PANI composite preparation:
[0043] (1), according to Ni 0.6 Zn 0.4 Fe 2 o 4 The stoichiometric ratio of Ni 2+ :Zn 2+ :Fe 3+ =3:2:10 Weigh nickel nitrate, zinc nitrate, ferric nitrate respectively, add distilled water, mix and dissolve completely under the condition of magnetic stirring, then press n(Fe 3+ ): n(CTAB)=2:1 Add surfactant cetyltrimethylammonium bromide (CTAB), and mix evenly under magnetic stirring; then slowly add the configured 3mol / L NaOH solution dropwise to In the above mixed solution, adjust the pH=9, react in a water bath at 60°C for 2 hours, and the solution is colloidal; wherein, adjust the pH to 10 or 11, react in a water bath at 80°C or 100°C for 4 hours or 6 hours, and obtain There was no significant difference in the experimental results.
[0044] (2) The solution was left to cool, and the supernatant was rem...
Embodiment 2
[0048] The molar ratio of aniline monomer to nickel zinc ferrite n(Ani):n(Ni 0.6 Zn 0.4 Fe 2 o 4 )=1:2 Ni 0.6 Zn 0.4 Fe 2 o 4 / PANI composite preparation:
[0049] (1), with (1) of embodiment 1
[0050] (2), (2) with embodiment 1
[0051] (3), with (3) of embodiment 1
[0052] (4) Preparation of Ni by in-situ polymerization 0.6 Zn 0.4 Fe 2 o 4 / PANI: Weigh aniline monomer and citric acid (CA) separately, add distilled water, mix well under mechanical stirring, then add a certain amount of nickel-zinc ferrite and mix under ice bath conditions, after mixing evenly, pour into the mixing Slowly add ammonium persulfate (APS) solution to the solution to initiate the reaction, and react under ice bath conditions, and the reaction time can be 6h-18h; after the reaction is completed, repeatedly wash with distilled water and absolute ethanol, vacuum Dry for 3 hours to obtain a nickel-zinc ferrite / polyaniline composite material; the molar ratio of the aniline monomer to citr...
Embodiment 3
[0054] The molar ratio of aniline monomer to nickel zinc ferrite n(Ani):n(Ni 0.6 Zn 0.4 Fe 2 o 4 )=1:3 Ni 0.6 Zn 0.4 Fe 2 o 4 / PANI composite preparation:
[0055] (1), with (1) of embodiment 1
[0056] (2), (2) with embodiment 1
[0057] (3), with (3) of embodiment 1
[0058] (4) Preparation of Ni by in-situ polymerization 0.6 Zn 0.4 Fe 2 o 4 / PANI: Weigh aniline monomer and citric acid (CA) separately, add distilled water, mix well under mechanical stirring, then add a certain amount of nickel-zinc ferrite and mix under ice bath conditions, after mixing evenly, pour into the mixing Slowly add ammonium persulfate (APS) solution to the solution to initiate the reaction, and react under ice bath conditions, and the reaction time can be 6h-18h; after the reaction is completed, repeatedly wash with distilled water and absolute ethanol, vacuum Dry for 3 hours to obtain a nickel-zinc ferrite / polyaniline composite material; the molar ratio of the aniline monomer to cit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com