Application of aryl naphthalene type lignan in preparation of medicine for resisting prostate cancer
An anti-prostate cancer, prostate cancer technology, applied in the field of medicine, to achieve strong pharmacological effects, inhibit proliferation, and good anti-prostate cancer activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
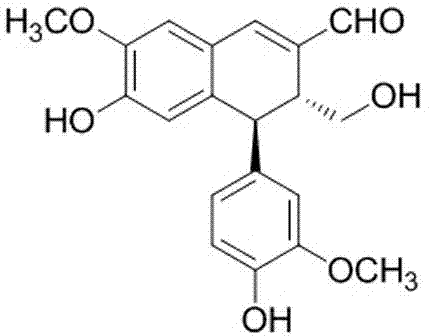
[0025] Embodiment 1: the preparation of aryl naphthalene type lignans
[0026] (1) Preparation of extraction solution: After crushing the medicinal materials of Vitex pratense, put them into the extraction tank, heat extraction with 40-90% ethanol for 2-3 times, the amount of solvent used each time is about 8-10 times the amount of crude drug, each extraction The time is 1 to 2 hours, and the extracts are combined;
[0027] (2) Refining, concentrating and drying: Concentrate the above extract under reduced pressure, recover the solvent, degrease the concentrated solution with petroleum ether, extract with ethyl acetate, and concentrate to obtain the ethyl acetate part;
[0028] (3) Separation and purification: Dissolve the above ethyl acetate part in water and load the macroporous adsorption resin AB-8. After the sample is adsorbed, wash with 5 times the volume of the column bed to remove impurities, and then sequentially use a volume fraction of 20 , 40, 60, 80, and 95% et...
Embodiment 2
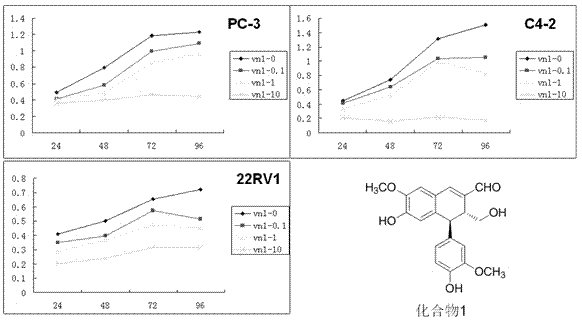
[0030] Example 2: Arylnaphthyl Lignans on Human Prostate Cancer Cells (PC-3 (hormone-independent prostate cancer cell line), 22RV1 (hormone-dependent prostate cancer cell line) and C4-2 (hormone-independent prostate cancer cell line)) proliferation effects
[0031] (1) Cell culture
[0032] The PC-3, 22RV1 and C4-2 cell culture system was RPMI1640 medium (Gibco), containing 10% fetal bovine serum (hyclone), 100 U / ml penicillin, 100 U / ml streptomycin at 37°C, 5% CO 2 cultured under saturated humidity conditions.
[0033] (2) Cell proliferation test
[0034] Using the Cell Counting Kit-8 kit (CCK-8), a certain number of PC-3, 22RV1 and C4-2 cells in the exponential growth phase were inoculated on a 96-well plate, and divided into blank control group and administration (the drug is 6-Hydroxy-4β-(4-hydroxy-3-methoxyphenyl)-3α-hydroxymethyl-7-methoxy-3,4-dihydro-2-naphthylaldehyde) group (0.1,1 and 10 μM) 6 replicate wells were set up for each group. The cells were incubated...
Embodiment 3
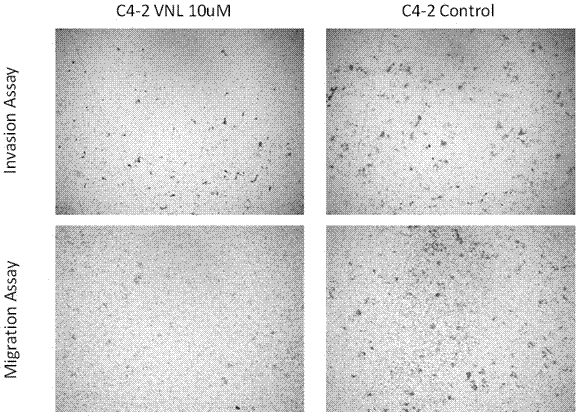
[0036] Example 3: Effects of Arylnaphthyl Lignans on Invasion and Migration of Human Prostate Cancer Cells (PC-3 and C4-2)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com