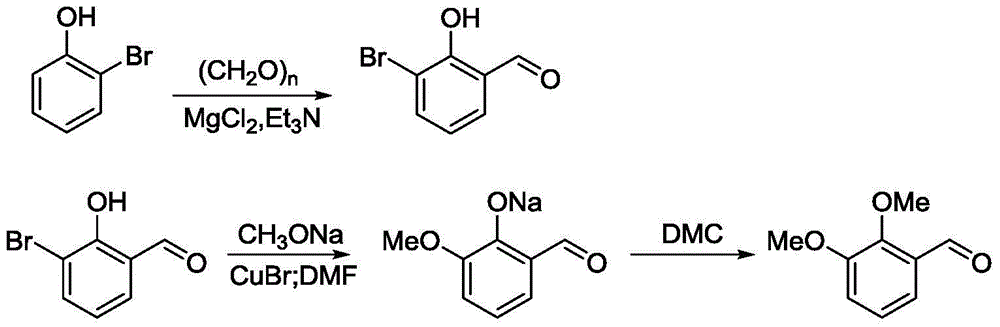
A kind of preparation method of 2,3-dimethoxybenzaldehyde
A technology of dimethoxybenzaldehyde and bromobenzaldehyde is applied in the preparation of pharmaceutical intermediates and in the field of preparation of 2,3-dimethoxybenzaldehyde, and can solve the problems of environmental pollution, high process cost and high toxicity. To achieve the effect of reducing environmental pollution, high product quality and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
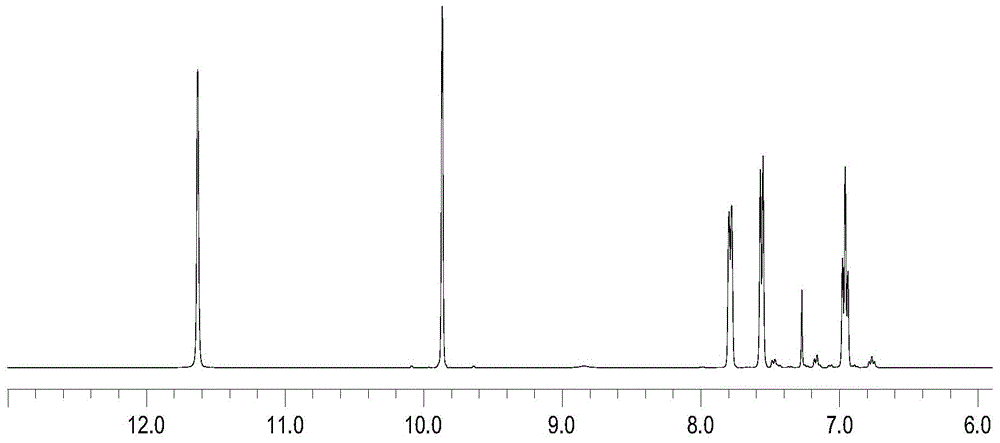
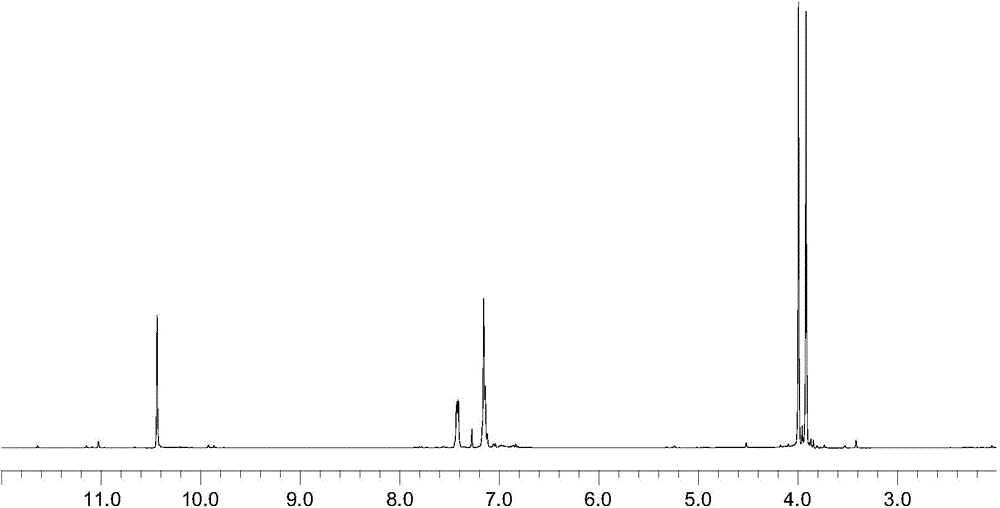
[0019] In a 250mL three-necked flask, stir magnetically, put 5.10g (0.03mol) of o-bromophenol, 1.78g (0.06mol) of paraformaldehyde, 5.50g (0.06mol) of anhydrous magnesium chloride, 5.85g (0.06mol) of triethylamine, toluene 60mL, reacted at 100°C for 6h. Cool to 50°C and add 10% hydrochloric acid to adjust the pH to 2-3, separate the liquid with a separatory funnel, dry the organic phase (upper layer) with anhydrous sodium sulfate until the liquid is clear without water drops, remove sodium sulfate by filtration, and distill After the solvent in the filtrate, distill under reduced pressure under 10mmHg pressure, collect 5.50g of fractions at 104~106°C, analyze as 2-hydroxyl-3-bromobenzaldehyde, and test the purity by gas chromatography with a purity of more than 99% (the same as in the following examples), Yield 92.9%.
Embodiment 2
[0021] In a 250mL three-necked flask, stir magnetically, put 5.19g (0.03mol) of o-bromophenol, 2.65g (0.09mol) of paraformaldehyde, 5.50g (0.06mol) of anhydrous magnesium chloride, 5.80g (0.06mol) of triethylamine, toluene 50mL, react at 100°C for 6h. Cool to 50°C and add 10% sulfuric acid to adjust the pH to 2-3, separate the liquid with a separatory funnel, dry the organic phase with anhydrous sodium sulfate, filter to remove sodium sulfate, distill off the solvent in the filtrate, and reduce it under a pressure of 10mmHg. Pressure distillation, collecting fractions at 104-106°C, yielded 5.78 g of 2-hydroxy-3-bromobenzaldehyde, with a yield of 94.6%.
Embodiment 3
[0023] In a 100mL three-neck flask, stir magnetically, put 1.70g (0.01mol) of o-bromophenol, 1.18g (0.04mol) of paraformaldehyde, 1.91g (0.02mol) of anhydrous magnesium chloride, 2.02g (0.02mol) of triethylamine, toluene 30mL, reacted at 100°C for 6h. Cool to 50°C and add 10% hydrochloric acid to adjust the pH to 2-3, separate the liquid with a separatory funnel, dry the organic phase with anhydrous sodium sulfate, filter to remove sodium sulfate, distill off the solvent in the filtrate, and reduce it under a pressure of 10mmHg. Pressure distillation, collecting fractions at 104-106°C, yielded 1.87 g of 2-hydroxy-3-bromobenzaldehyde with a yield of 97.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com