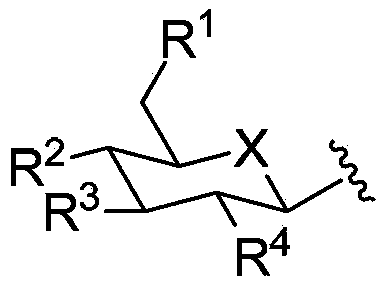
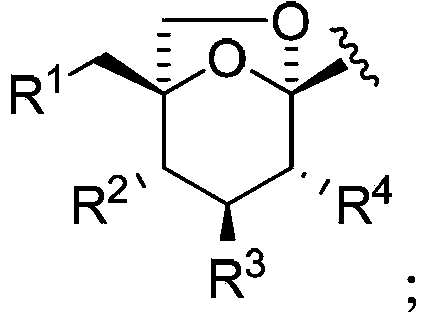
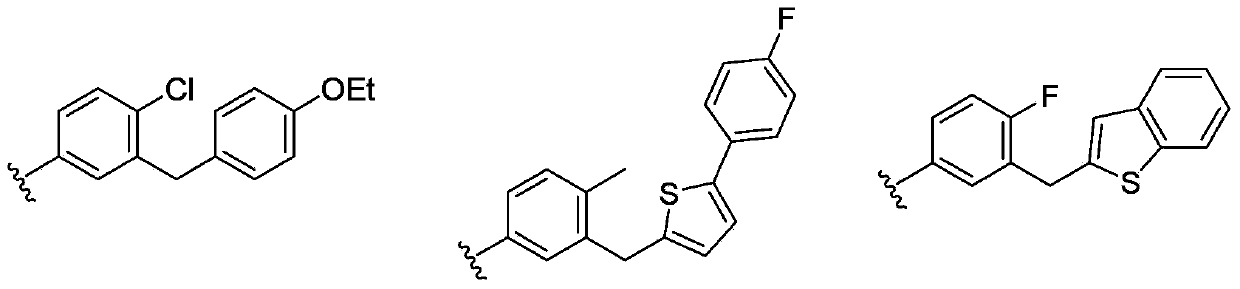
Phenyl C-glucoside derivative containing deoxyglucose structure as well as preparation method and application thereof
A -CH2-O-, alkyl technology, applied in the field of diabetes-related drugs, can solve problems such as difficulty in reaching blood sugar control goals, weight gain, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0144] Preparation of (1S)-1-[4-chloro-3-(4-ethoxybenzyl)phenyl]-1,6-dideoxy-D-glucose (I-D1-6)
[0145]
[0146]
[0147] a.
[0148] 4.09g (10mmol) of compound 1 was dissolved in 30mL of dry DMF, stirred under cooling in an ice-water bath, 2.72g (40mmol) of imidazole was added, and then 1.66g (11mmol) of TBDMSCl (tert-butyl dimethyl silicon chloride). After the addition was complete, the reaction mixture was stirred for an additional 3 hours at room temperature. The reaction mixture was diluted with 150 mL of dichloromethane, washed with 50 mL×3 saturated brine, and dried over anhydrous sodium sulfate. The desiccant was removed by filtration, the solvent was evaporated from the filtrate on a rotary evaporator, and the obtained residue was subjected to silica gel column chromatography to obtain pure product 2 as a white foamy solid. 1 H NMR (DMSO-d 6 ,400MHz),δ7.35(d,1H,J=8.0Hz),7.28(d,1H,J=2.0Hz),7.17(dd,1H,J=2.0Hz and8.4Hz),7.05(d,2H ,J=8.8Hz),6.79(d,2H,J=8.8Hz),...
Embodiment 2
[0160] Preparation of (1S)-1-[4-chloro-3-(4-ethoxybenzyl)phenyl]-1,4-dideoxy-D-glucose (I-D1-4)
[0161]
[0162] a.
[0163] 4.09 g (10 mmol) of compound 1, 1.83 g (12 mmol) of benzaldehyde dimethyl acetal and 0.1 g of CAS (camphorsulfonic acid) were dissolved in 30 mL of dry DMF, and heated and stirred at 110° C. for 3 hours under a nitrogen atmosphere. After cooling, the reaction mixture was diluted with 150 mL of dichloromethane, washed successively with 20 mL of 5% sodium carbonate solution and saturated brine, and dried over anhydrous sodium sulfate. The desiccant was removed by filtration, the solvent was evaporated from the filtrate on a rotary evaporator, and the obtained residue was subjected to silica gel column chromatography to obtain pure product 7 as a white solid. Melting point 176-178°C. 1 H NMR (DMSO-d 6 ,400MHz),δ7.45-7.47(m,2H),7.36-7.40(m,4H),7.28(d,1H,J=1.6Hz),7.21(dd,1H,J=2.0Hz and8.4Hz) ,7.08(d,2H,J=8.8Hz),6.83(d,2H,J=8.4Hz),5.60(s,1H),5.31(d,1H,...
Embodiment 3
[0177] Preparation of (1S)-1-[4-chloro-3-(4-ethoxybenzyl)phenyl]-1,3-dideoxy-D-glucose (I-D1-3)
[0178]
[0179] a.
[0180] 4.97g (10mmol) of compound 7 was dissolved in 30mL of dry DMF, stirred in an ice-water bath, 2.72g (40mmol) of imidazole was added, and then 1.66g (11mmol) of TBDMSCl (tert-butyl dimethyl silicon chloride). After the addition was complete, the reaction mixture was stirred for an additional 3 hours at room temperature. The reaction mixture was diluted with 150 mL of dichloromethane, washed with 50 mL×3 saturated brine, and dried over anhydrous sodium sulfate. The desiccant was removed by filtration, the filtrate was evaporated on a rotary evaporator to remove the solvent, and the obtained residue was subjected to silica gel column chromatography to obtain pure product 13 as a white foamy solid. ESI-MS, m / z=628 ([M+NH 4 ] + ).
[0181] b.
[0182] 4.89g (8mmol) of compound 13 was dissolved in 30mL of pyridine, and stirred under cooling in an ice...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com