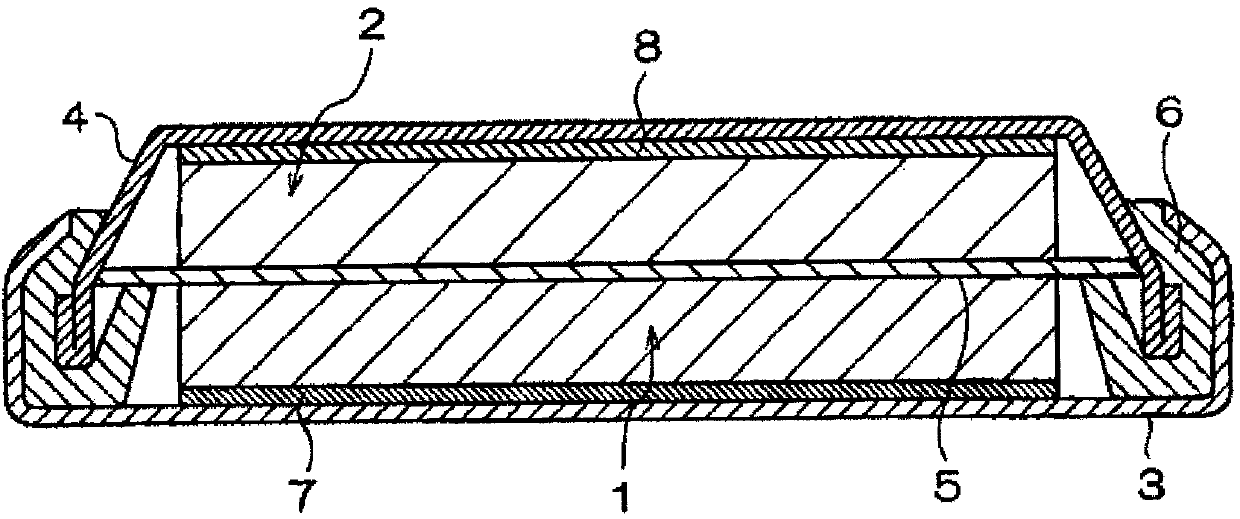
Nonaqueous electrolyte solution containing phosphonosulfonic acid compound, and lithium secondary battery
一种非水电解液、膦酰基磺酸的技术,应用在膦酰基磺酸化合物领域,能够解决不能获得电池低温放电特性改善电池保存特性改善等问题,达到保存特性改善、低温放电特性改善的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0291] The following examples illustrate the present invention more specifically, but the present invention is not limited by these examples. It should be noted that, in the following examples, "%" or "wt%" means mass %.
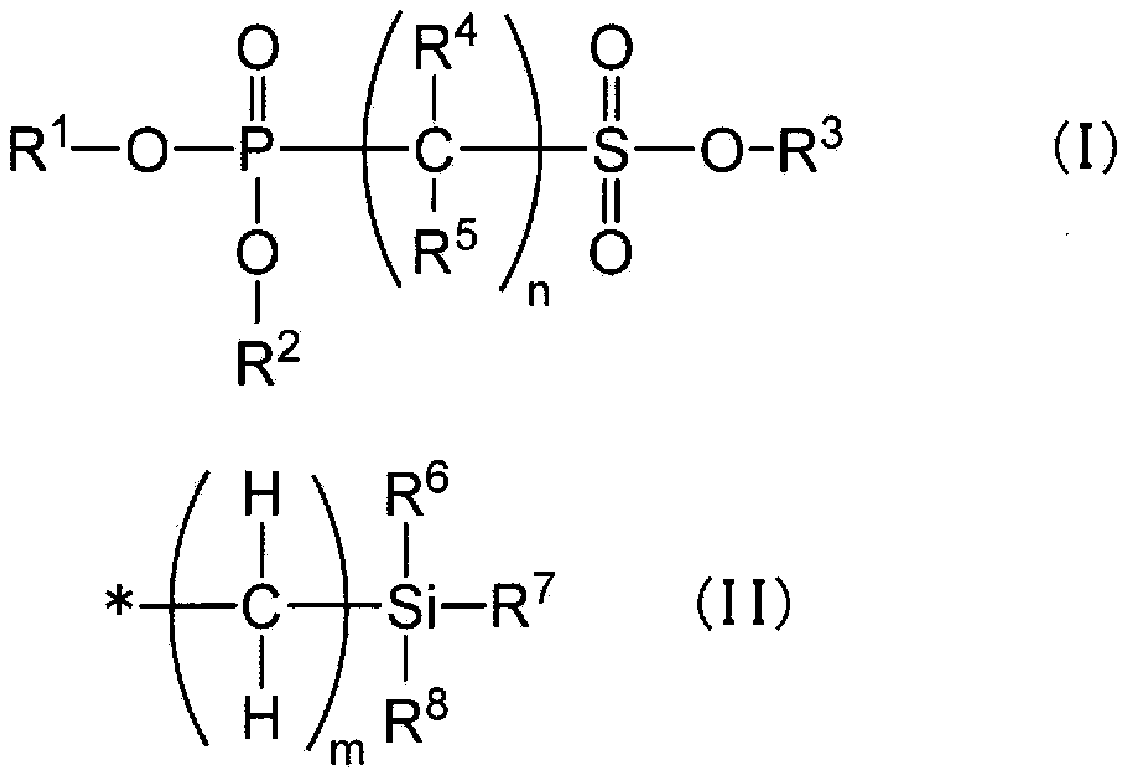
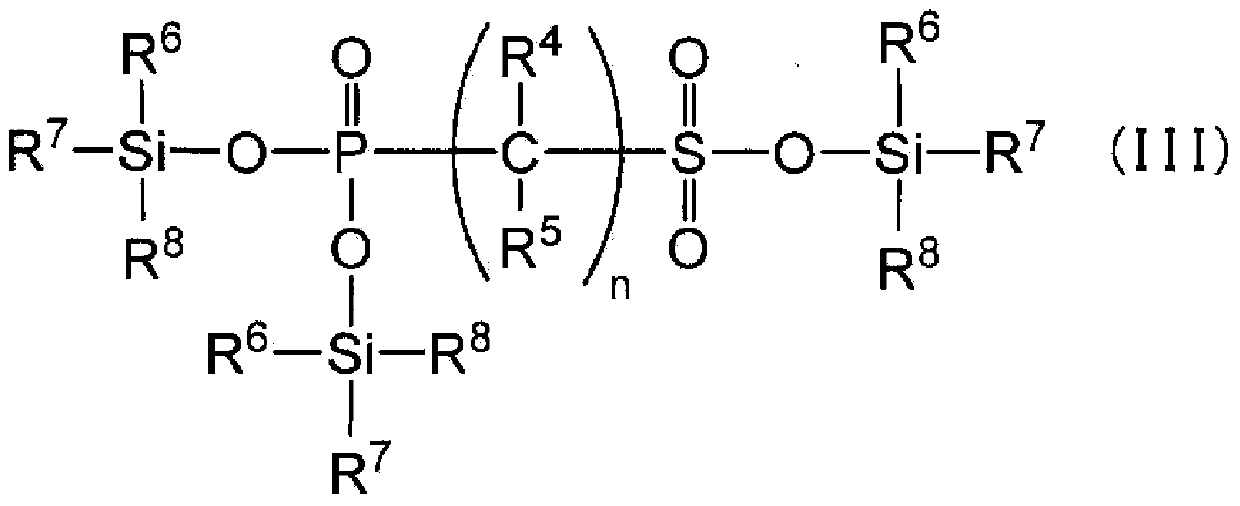
[0292] Synthesis examples of phosphonosulfonic acid compounds represented by the general formula (I) are given below.
Synthetic example 1
[0294]
[0295] Dissolve methyl methanesulfonate (5.00g, 45.4mmol) in tetrahydrofuran (100ml), cool to -78°C, add dropwise n-butyllithium (1.6M hexane solution, 31ml, 49.9mmol), and at the same temperature Stir for 30 minutes. Next, diethyl chlorophosphate (3.9 ml, 27.2 mmol) was added dropwise, followed by stirring at -78°C for 1 hour and then at -50°C for 30 minutes. After adding saturated aqueous ammonium chloride solution to the reaction liquid and stirring, it was further diluted with water, and the diluted mixture was extracted twice with ethyl acetate. The combined extract (organic layer) was washed with water and saturated brine, dried over anhydrous magnesium sulfate, and concentrated. The obtained oil was purified by silica gel column chromatography (ethyl acetate / hexane system) to obtain 5.04 g (yield 75%) of methyl diethylphosphonomethanesulfonate (exemplary compound 10). The NMR measurement results of the obtained compound are as follows.
[0296] 1 H-NMR (2...
Synthetic example 2
[0298]
[0299] Methyl diethylphosphonomethanesulfonate (exemplary compound 10) (5.04 g, 20.5 mmol) was dissolved in dichloromethane (25 ml), and bromotrimethylsilane (10.8 ml, 80.9 mmol) was added at room temperature. After stirring at room temperature for 6 hours, the reaction solution was concentrated under reduced pressure to obtain trimethylsilyl bis(trimethylsilyl)phosphonomethanesulfonate (exemplary compound 23) (7.67 g, yield 95%). The NMR measurement results of the obtained compound are as follows.
[0300] 1 H-NMR (270MHz, CDCl 3 ) δ (ppm): 3.90 (2H, d, J = 18.4Hz), 0.36 (18H, s), 0.07 (9H, s).
PUM
| Property | Measurement | Unit |
|---|---|---|
| actual density | aaaaa | aaaaa |
| packed density | aaaaa | aaaaa |
| packed density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com