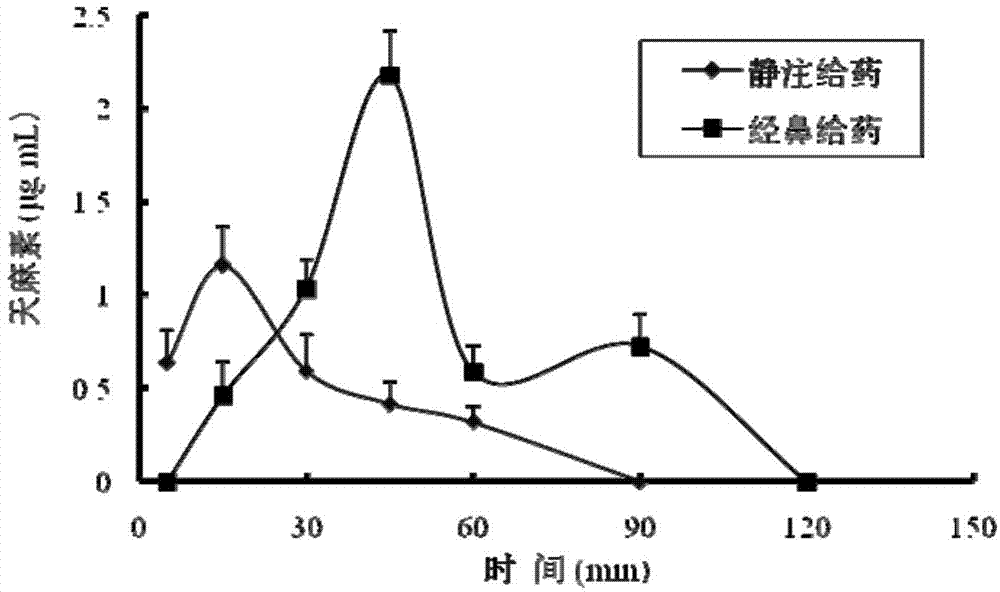
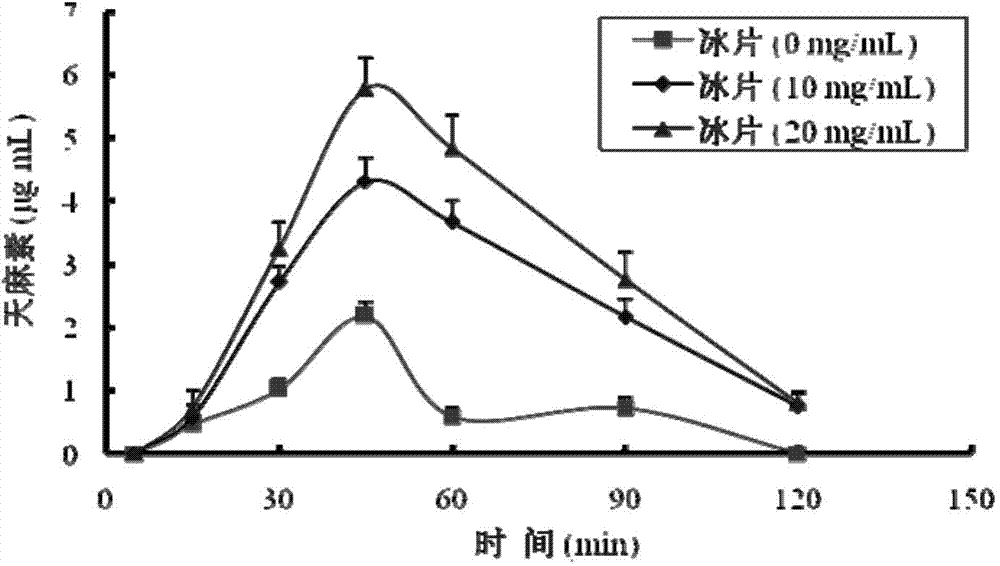
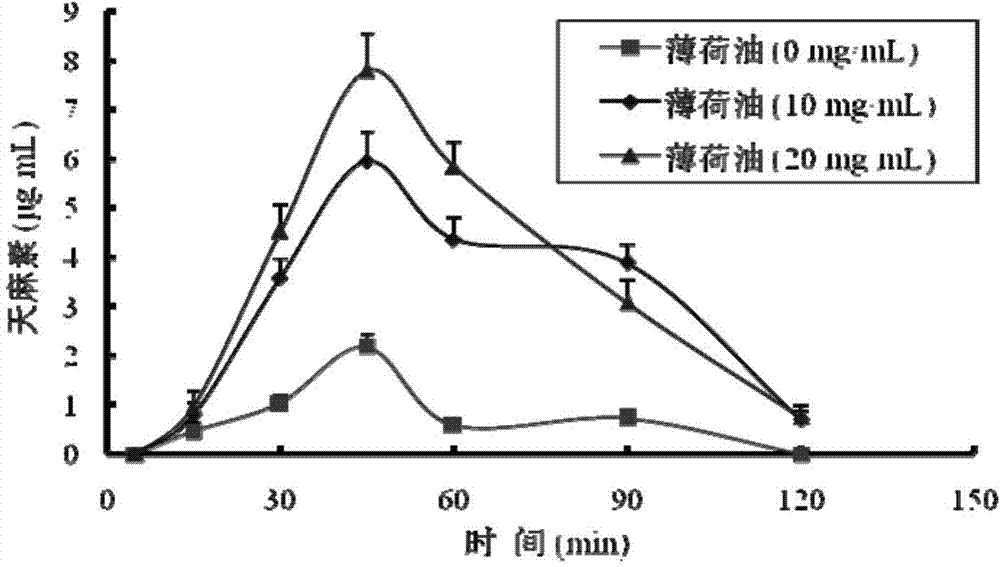
Gastrodin in-situ gel for treating inner ear diseases
A technology of gastrodin and gel, which is applied in the field of pharmaceutical preparations containing organic active ingredients, can solve the problems of negligible dosage and difficulty in reaching the labyrinth area of the inner ear, and achieve the goals of promoting efficiency, improving bioavailability, and prolonging residence time Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] 1. Prescription: Gastrodin 10g, deacetylated gellan gum 0.5g, mint oil 1g, ethylparaben 0.03g, purified water 100g.
[0037] 2. Preparation method: Weigh deacetylated gellan gum and ethyl paraben, add 80g of purified water, dissolve at 90°C and let cool to room temperature, add gastrodin, refrigerate the dissolved solution at 4°C for 24 hours, and then pass through Filtrate through a fused glass funnel, add peppermint oil and the remaining purified water, stir evenly, and divide into packages to obtain the product.
Embodiment 2
[0039] 1. Prescription: gastrodin 20g, sodium alginate 0.8g, borneol 2g, benzalkonium chloride 0.02g, purified water 100g.
[0040] 2. Preparation method: Weigh sodium alginate and benzogadolinium ammonium chloride, add 80g of purified water, dissolve at 90°C and let cool to room temperature, then add gastrodin, the dissolved solution is refrigerated at 4°C for 24 hours and then vertically melted Filter through a glass funnel, add borneol fine powder, then add the remaining purified water to a sufficient amount, stir evenly, and divide into packages to obtain.
Embodiment 3
[0042] 1. Prescription: Gastrodin 10g, deacetylated gellan gum 0.3g, peppermint oil 0.5g, ethylparaben 0.05g, purified water 100g.
[0043] 2. Preparation method: Weigh deacetylated gellan gum and ethyl paraben, add 80g of purified water, dissolve at 90°C and let cool to room temperature, then add gastrodin, refrigerate the dissolved solution at 4°C for 24 hours, and then Filter through a vertically fused glass funnel, add peppermint oil, and then add the remaining purified water to a sufficient amount, stir evenly, and pack separately to obtain.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com