Visible-light responsive magnetic photocatalytic material and preparation method thereof
A photocatalytic material, visible light technology, applied in the field of photocatalytic materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
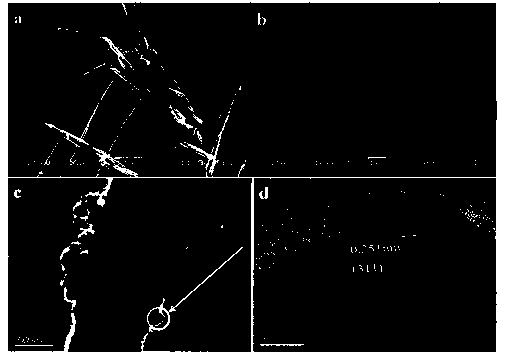
[0024] (1) Preparation of nickel ferrite: fully dissolve nickel nitrate and ferric nitrate in deionized water, wherein the ratio of nickel nitrate and ferric nitrate is 1:4, adjust the pH with 6mol / L sodium hydroxide solution to 14 to obtain mixed solution A. Then transfer the mixed solution A to the reaction kettle, the preferred reaction kettle is a stainless steel reaction kettle with polytetrafluoroethylene liner, the set reaction temperature is 180°C, 200°C, 220°C, 240°C, and the reaction time is 24 h. After the reaction, cool down to room temperature, the cooling method is natural cooling, or other common physical cooling methods, wash with deionized water and ethanol, and dry by vacuum drying or natural drying to obtain nickel ferrite powder. The morphology of the SEM image was investigated, and when the reaction temperature was 220°C, the morphology of the nickel ferrite was the most regular, which was a nano-sized cube.
[0025] (2) Compounding of nickel ferrite and ...
Embodiment 2
[0027] (1) Preparation of nickel ferrite: fully dissolve nickel nitrate and ferric nitrate in deionized water, wherein the amount of nickel nitrate and ferric nitrate is 1:4, and adjust the pH with 6mol / L sodium hydroxide solution to 14. Obtain mixed solution A. Then transfer the mixed solution A to the reaction kettle, the preferred reaction kettle is a stainless steel reaction kettle with a polytetrafluoroethylene liner, the set reaction temperature is 220°C, the reaction time is 12 h, 24 h, 36 h, after the reaction is completed , cooled to room temperature, the cooling method is natural cooling, or other common physical cooling methods, washed with deionized water and ethanol, and dried by vacuum drying or natural drying to obtain nickel ferrite powder, which is inspected by taking SEM pictures Among them, when the reaction temperature is 24 h, the morphology of nickel ferrite is the most regular, which is a nano-sized cube.
[0028] (2) Compounding of nickel ferrite and b...
Embodiment 3
[0030] (1) Preparation of nickel ferrite: fully dissolve nickel nitrate and ferric nitrate in deionized water, wherein the amount of nickel nitrate and ferric nitrate is 1:4, and adjust the pH with 6mol / L sodium hydroxide solution to 14. Obtain mixed solution A. Then transfer the mixed solution A to the reactor, the preferred reactor is a stainless steel reactor with a polytetrafluoroethylene liner, the reaction temperature is 220°C, and the reaction time is 24 h. After the reaction is completed, cool to room temperature, and the cooling method is natural Cooling, or other common physical cooling methods, washing with deionized water and ethanol, and drying by vacuum drying or natural drying, the nickel ferrite powder is obtained.
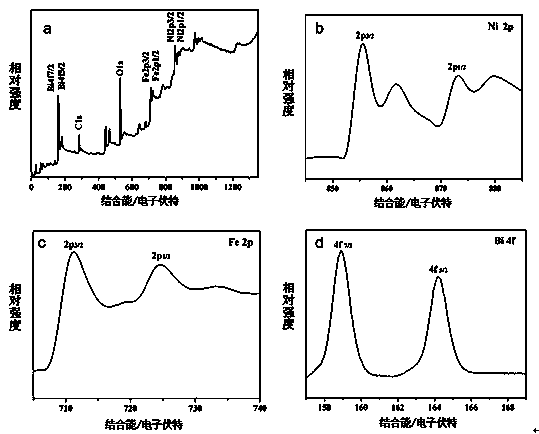
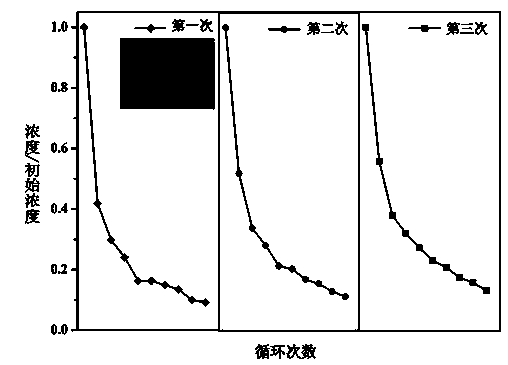
[0031] (2) Compounding of nickel ferrite and bismuth oxide: Dissolve 0.970g of bismuth nitrate, 0.426g of sodium sulfate and 0.936g of nickel ferrite in 40 ml of deionized water. For solution B, dissolve 0.720g of sodium hydroxide in 40mL of deion...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com