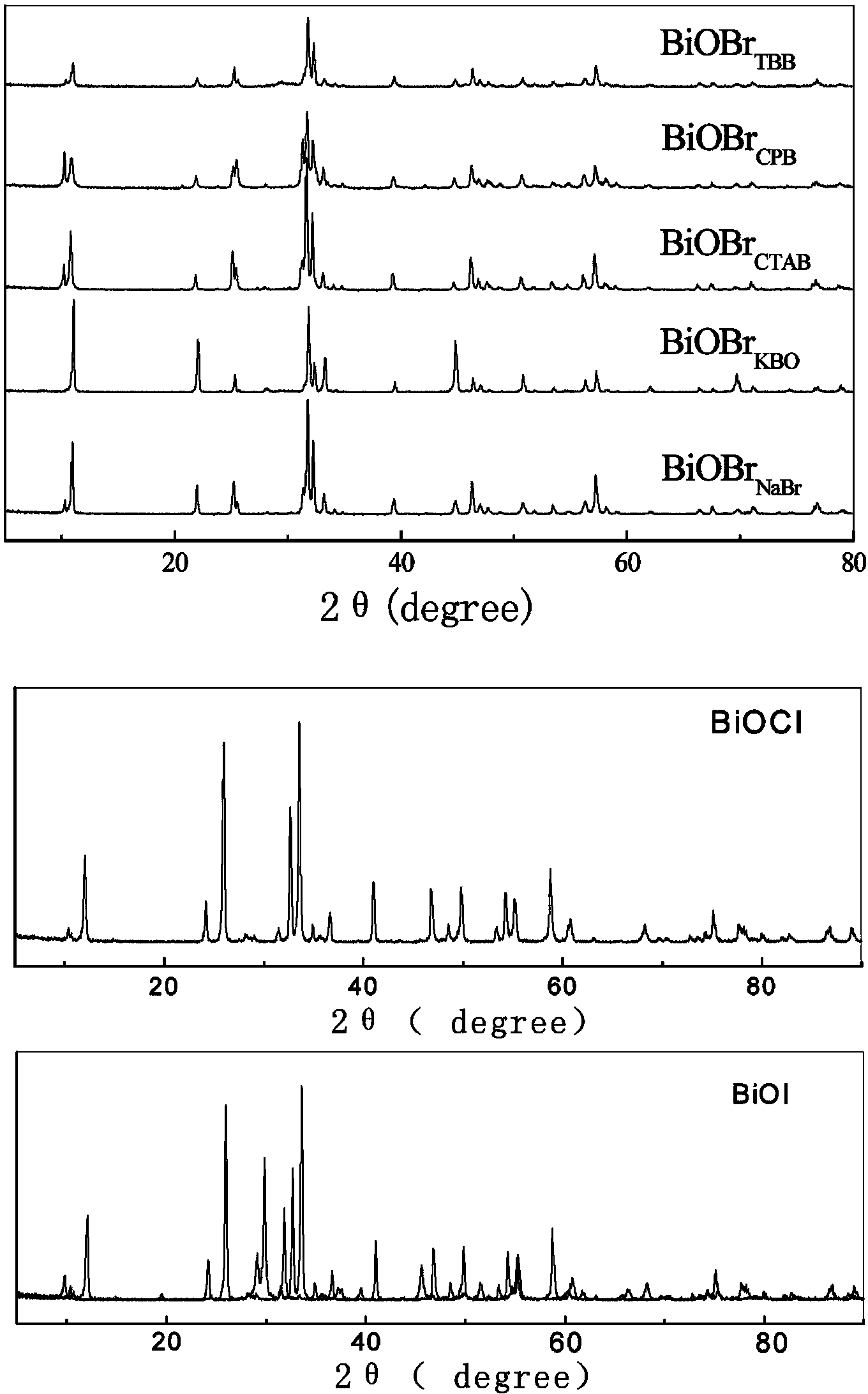
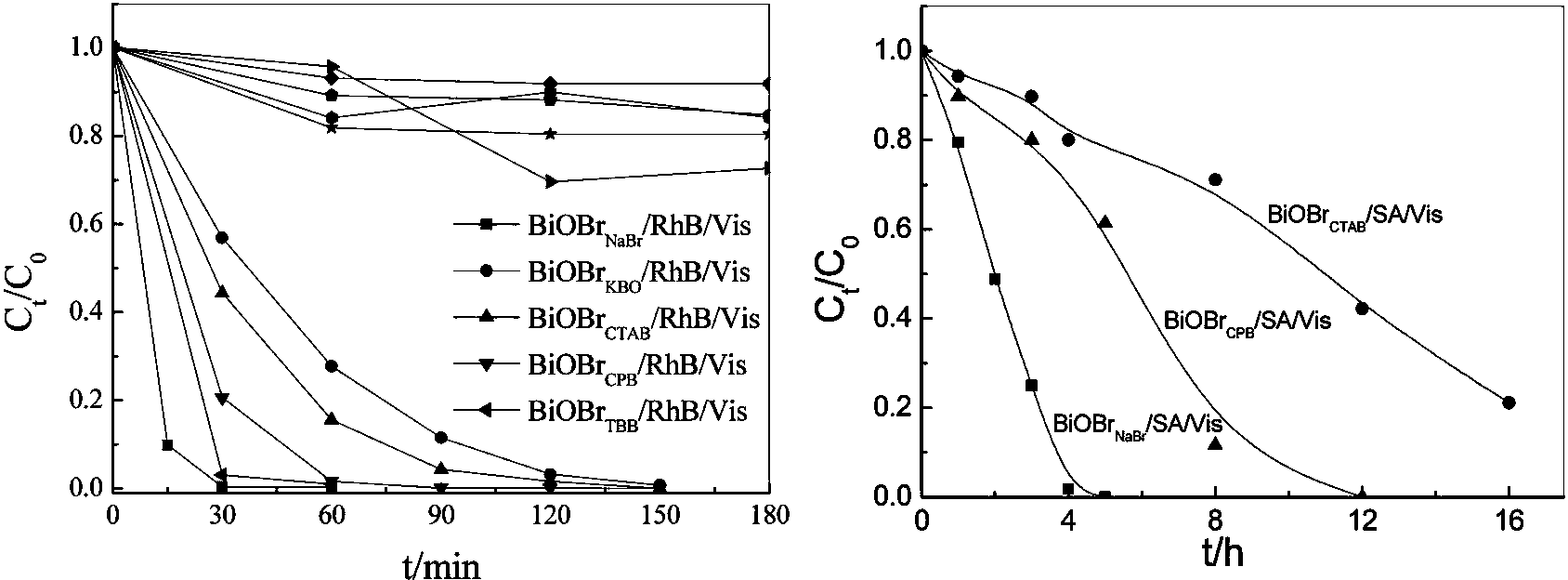
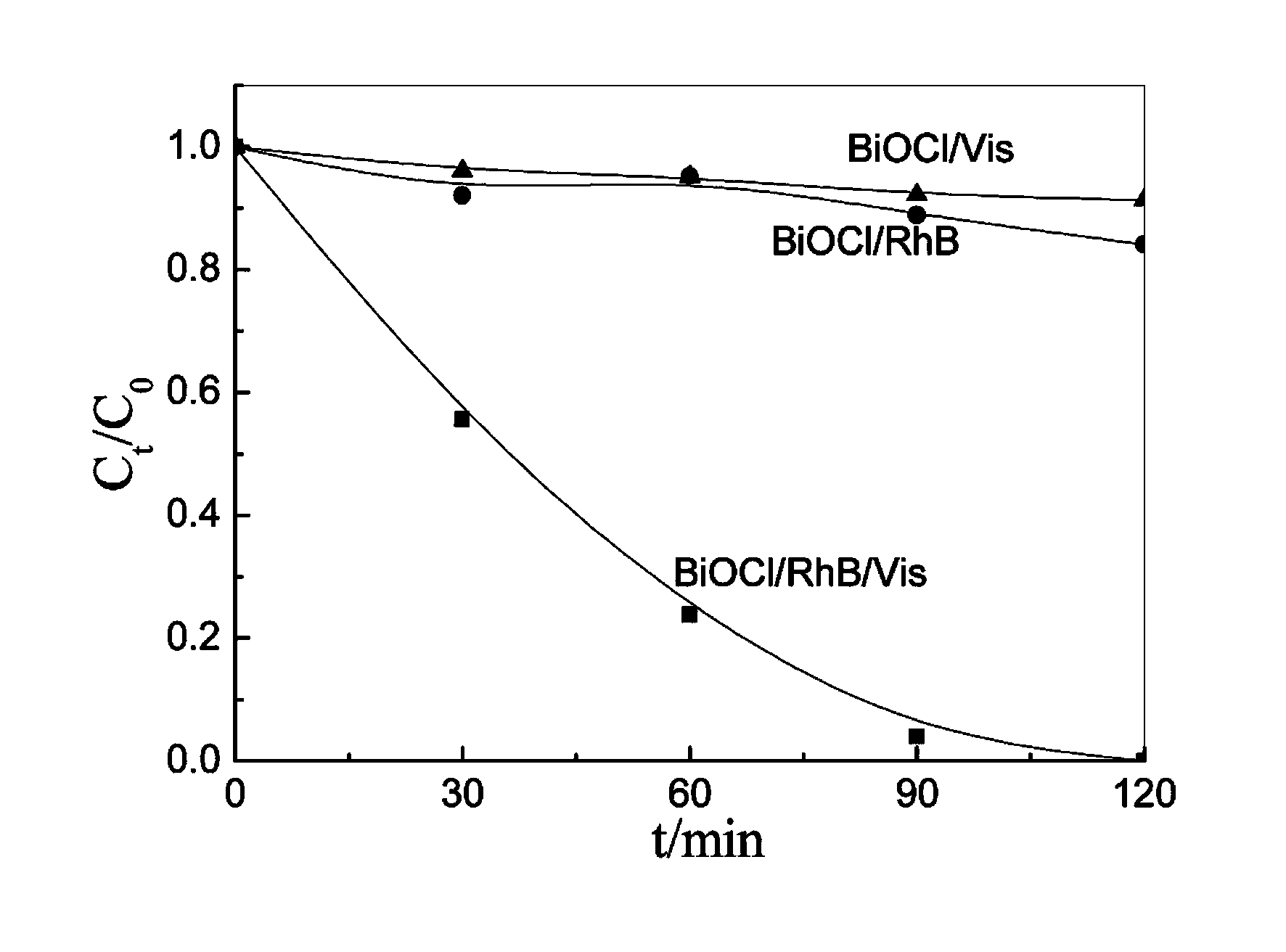
Bismuth oxyhalide photocatalyst and preparation method thereof
A bismuth oxyhalide, photocatalyst technology, applied in physical/chemical process catalysts, chemical instruments and methods, inorganic chemistry, etc., can solve problems such as secondary pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Dissolve 0.001 mol of sodium bromide in 70 mL of deionized water, and add 0.001 mol of sodium bismuthate. Adjust pH=1.16. Place in a 100 mL polytetrafluoroethylene reactor and react at 180°C for 24 h. After the reaction is complete, the resulting precipitate is suction-filtered, washed with alcohol and water respectively, and dried at 60°C for 4 h.
Embodiment 2
[0019] Take 0.001 mol of cetylpyridine bromide and dissolve it in 70 mL of deionized water, and add 0.001 mol of sodium bismuthate. Adjust pH=1.06. Place in a 100 mL polytetrafluoroethylene reactor and react at 180°C for 12 h. After the reaction is complete, the resulting precipitate is suction-filtered, washed with alcohol and water respectively, and dried at 60°C for 4 h.
Embodiment 3
[0021] Dissolve 0.001 mol of cetyltrimethylammonium bromide in 70 mL of deionized water, dissolve it by ultrasonication, and add 0.001 mol of sodium bismuthate. Adjust pH=1.26. Place in a 100 mL polytetrafluoroethylene reactor and react at 160°C for 12 h. After the reaction is complete, filter the resulting precipitate with suction, wash with alcohol and water respectively, and dry at 60°C for 4 h.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com