Preparation method of ezetimibe chiral intermediate
A chiral catalyst and compound technology, which is applied in the field of preparation of ezetimibe chiral intermediates, can solve the problems of low yield, low atom economy, complicated post-processing and the like, and achieves low dosage and high application value. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
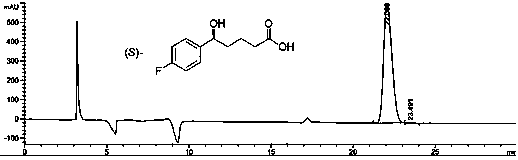
[0064] Embodiment 1: ( S Preparation of )-5-(4-fluorophenyl)-5-hydroxypentanoic acid
[0065] Weigh (5.0 mg, 0.005 mmol) chiral catalyst M (X is 3-methyl) and (30.3 g, 270 mmol) potassium tert-butoxide into the inner tube of the reaction, put the inner tube into the autoclave, Add 500 mL of ethanol and (52.6 g, 250 mmol) 5-(4-fluorophenyl)-5-oxopentanoic acid into the reaction inner tube, replace the gas in the kettle body with hydrogen, keep the hydrogen pressure at 0.2-10 MPa, and heat to The reaction was carried out at 50°C. After reacting for 10 hours, the reaction solution was concentrated. Add 300 mL of water and concentrated hydrochloric acid to the system to adjust pH=3~4. Add 300 mL of ethyl acetate, separate the layers, wash the organic phase with saturated brine, and dry over anhydrous sodium sulfate. Suction filtration, concentrated to light yellow solid ( S )-5-(4-fluorophenyl)-5-hydroxypentanoic acid 53.0 g, the yield was 100%, and the raw material 5-(4-fl...
Embodiment 2
[0074] Embodiment 2: ( S Preparation of )-5-(4-fluorophenyl)-5-hydroxypentanoic acid
[0075] Weigh (1.0 mg, 0.001 mmol) chiral catalyst M (X is H) and (606 mg, 5.4 mmol) potassium tert-butoxide into the reaction tube, put the reaction tube into the autoclave, Add 5 mL of ethanol and (1.05 g, 5 mmol) 5-(4-fluorophenyl)-5-oxopentanoic acid into the tube, replace the gas in the kettle with hydrogen, keep the hydrogen pressure at 0.2-10 MPa, and heat to 50°C react. After reacting for 20 hours, the reaction solution was concentrated. Add 10 mL of water and concentrated hydrochloric acid to the system to adjust pH=3~4. Add 10 mL of ethyl acetate, separate the layers, wash the organic phase with saturated brine once more, and dry over anhydrous sodium sulfate. Suction filtration, the solvent was concentrated to obtain a light yellow solid ( S )-5-(4-fluorophenyl)-5-hydroxypentanoic acid 1.06 g, the yield was 96.1%. According to the analysis by H NMR spectrum, the conversion o...
Embodiment 3
[0076] Embodiment 3: ( S Preparation of )-5-(4-fluorophenyl)-5-hydroxypentanoic acid
[0077] Weigh (1.0 mg, 0.001 mmol) chiral catalyst M (X is 4-methyl) and (40 mg, 1.0 mmol) potassium hydroxide into the reaction tube, put the reaction tube into the autoclave, Add 5 ml DMF and (10.5 g , 50 mmol) 5-(4-fluorophenyl)-5-oxopentanoic acid into the inner tube of the reaction, replace the gas in the kettle body with hydrogen, keep the hydrogen pressure at 0.2-10 MPa, and heat to The reaction was carried out at 50°C. After reacting for 20 hours, the reaction solution was concentrated. Add 10 mL of water and concentrated hydrochloric acid to the system to adjust pH=3~4. Add 10 mL of ethyl acetate, separate the layers, wash the organic phase with saturated brine once more, and dry over anhydrous sodium sulfate. Suction filtration, the solvent was concentrated to obtain a light yellow solid ( S )-5-(4-fluorophenyl)-5-hydroxypentanoic acid 10.3 g, the yield was 97.2%. According t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com