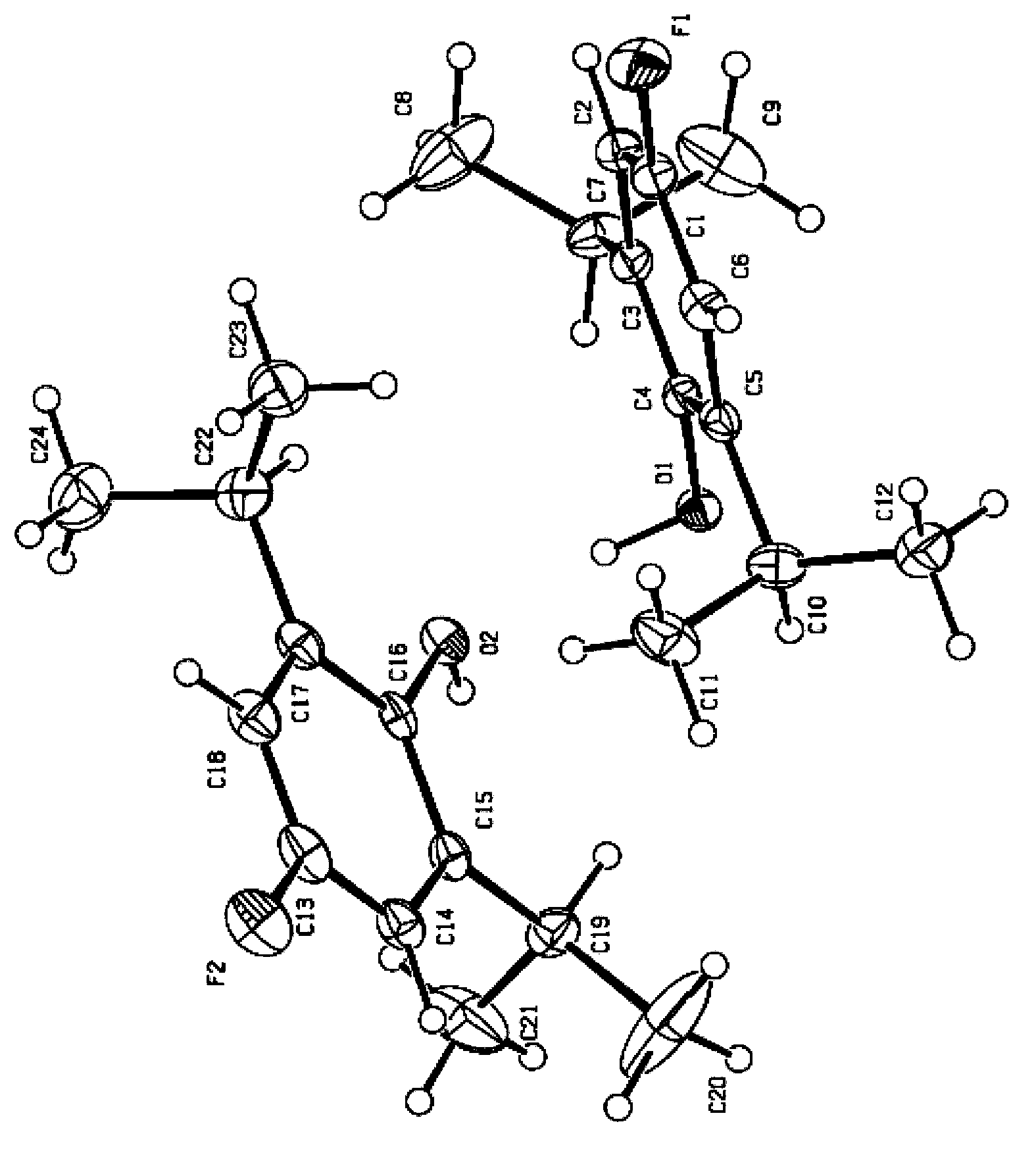
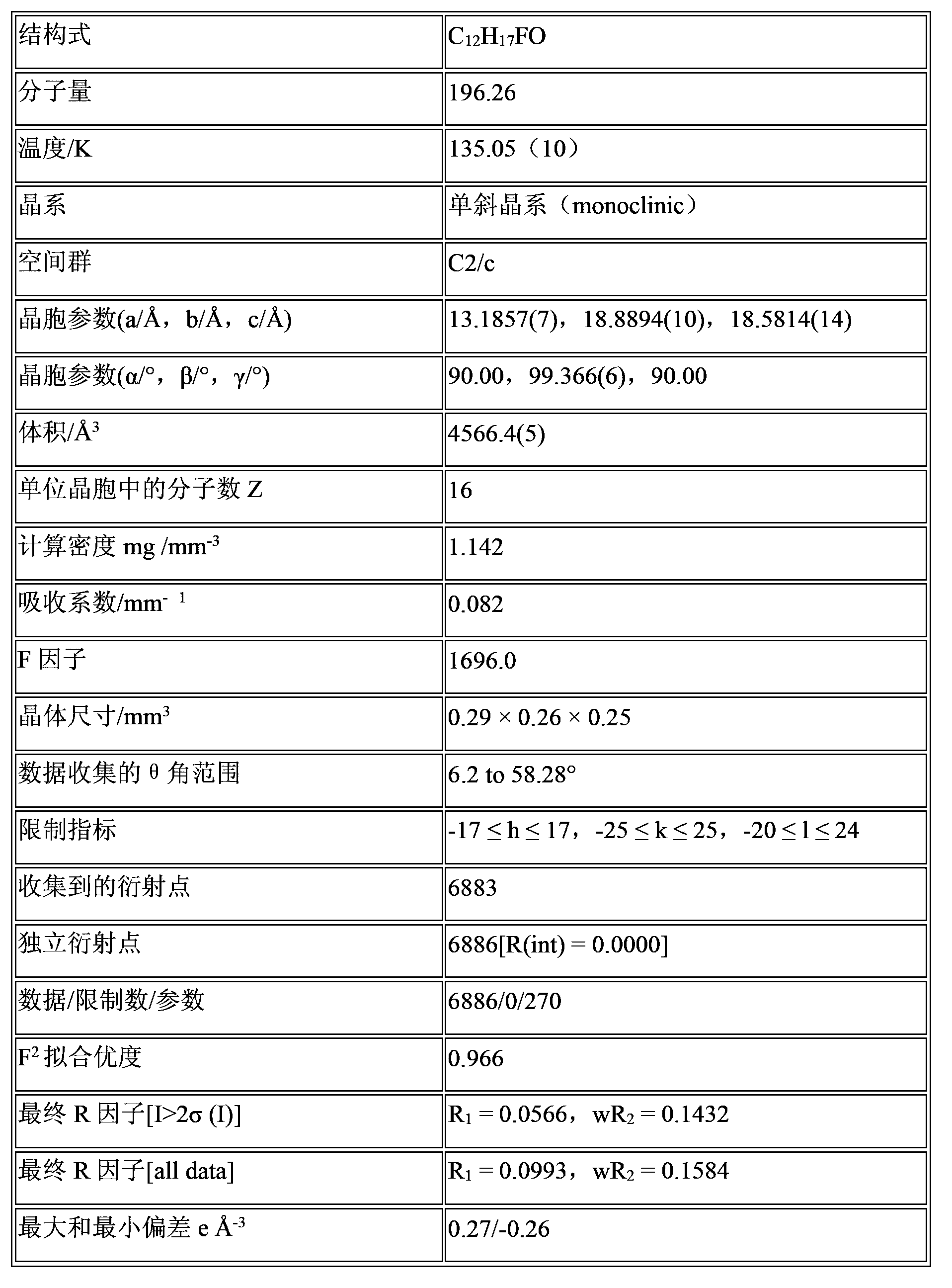
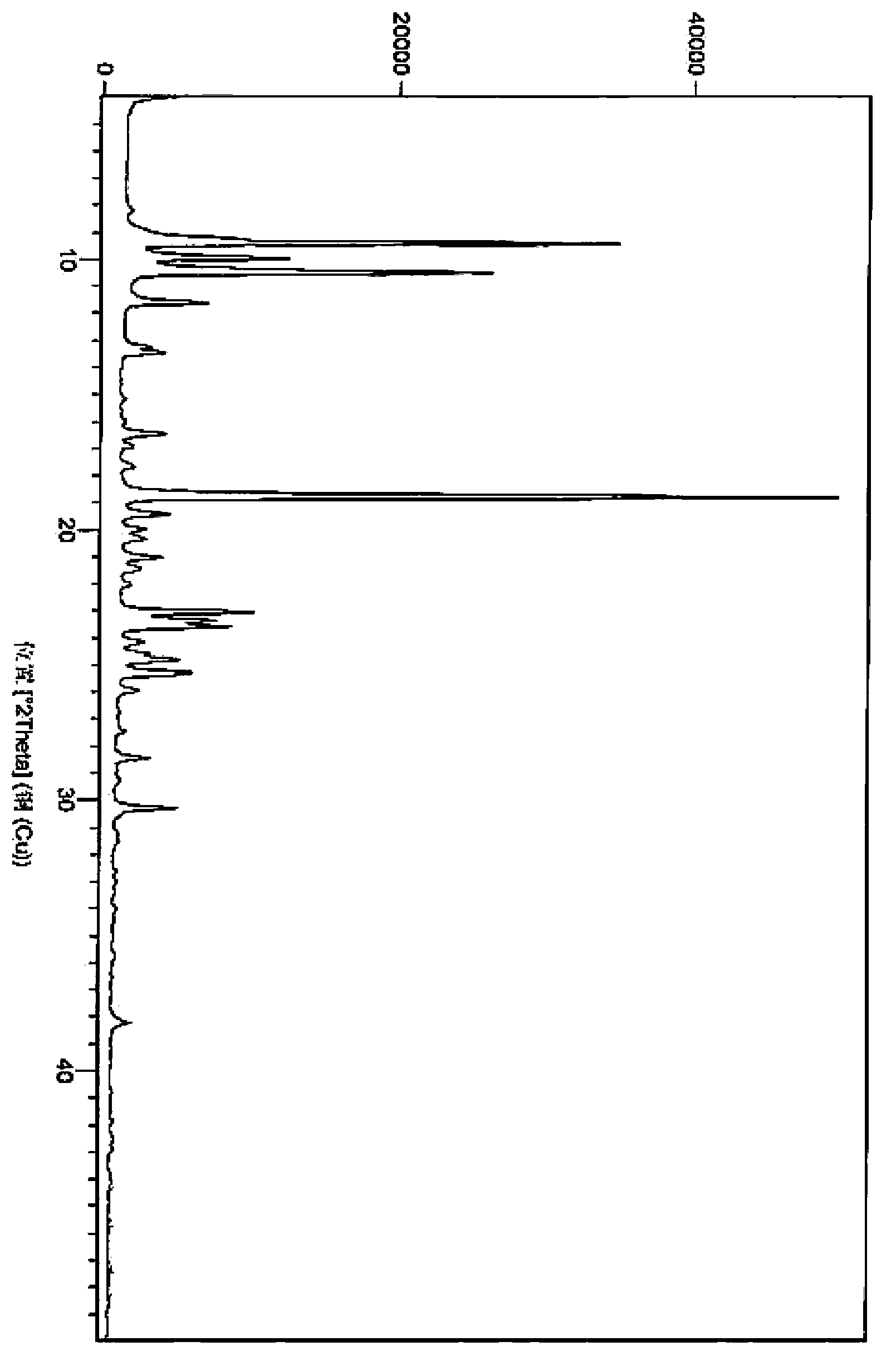
2,6-diisopropyl-4-fluorophenol or salt and crystal form thereof as well as preparation method and application thereof
A technology of diisopropyl and fluorophenol, which is applied in the field of medicine, can solve the problems of toxic side effects, easy oxidation, deterioration, etc., and achieve the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1: Synthesis of 2,6-Diisopropyl-4-bromoanisole (Formula 3)
[0054] Add 15L of acetone, 1440g of 2,6-diisopropyl-4-bromophenol, 867g of methyl iodide, and 345g of potassium hydroxide to a 25L reaction flask, and heat to reflux. After TLC shows the reaction is complete, stop heating, filter, and concentrate ,Extract with n-hexane 2L×3, wash with water to neutral, anhydrous Na 2 SO 4 The organic phase was dried and concentrated to obtain 1500 g of a colorless oil, which was 2,6-diisopropyl-4-bromoanisole. Yield: 99%.
[0055] The structural characterization data of 2,6-diisopropyl-4-bromoanisole are as follows:
[0056] GC-MS: m / z=270(M + );272(M+2) + .
[0057] 1 H NMR(CDCl 3 ,δ / ppm): 1.21-1.23(d, 12H, -CH 3 );3.25-3.32(m, 2H, -CH-); 3.71(s, 3H, -OCH 3 ); 7.19(s, 2H, -ArH).
[0058] The above data confirms that the product obtained is 2,6-diisopropyl-4-bromoanisole.
Embodiment 2
[0059] Example 2: Synthesis of 2,6-Diisopropyl-4-bromoanisole (Formula 3)
[0060] Add 3L of 1,4-dioxane, 288g of 2,6-diisopropyl-4-bromophenol, 173g of methyl iodide, and 69g of potassium hydroxide to a 5L reaction flask. Heat to reflux. TLC shows that the reaction is complete. , Stop heating, filter, concentrate, extract with n-hexane 700mL × 3, wash with water to neutral, anhydrous Na 2 SO 4 The organic phase was dried and concentrated to obtain 182 g of a colorless oil, which was 2,6-diisopropyl-4-bromoanisole. Yield: 60%.
[0061] The characterization results of the product were the same as in Example 1.
Embodiment 3
[0062] Example 3: Synthesis of 2,6-Diisopropyl-4-bromoanisole (Formula 3)
[0063] Add 50g of 2,6-diisopropyl-4-bromophenol, 3.1g of tetrabutylammonium bromide, 280ml of dimethyl carbonate and 40g of potassium carbonate to a 500mL three-necked flask. Heat to reflux. TLC shows that the reaction is complete. , Stop heating, extract with n-hexane 400mL×3, wash with water to neutral, anhydrous Na 2 SO 4 The organic phase was dried and concentrated to obtain 20 g of a colorless oil. Yield: 38%.
[0064] The characterization results of the product were the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com