Lithium battery positive electrode material and preparation method thereof
A cathode material, lithium battery technology, applied in battery electrodes, secondary batteries, circuits, etc., can solve the problem that nickel-cobalt-manganese ternary materials are difficult to achieve cycle performance, thermal stability performance and electrochemical performance, etc. The effect of low cost, high cycle performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0018] The invention provides a method for preparing a positive electrode material of a lithium battery, comprising the following steps:
[0019] S1. Pump solution A and alkali solution into the reaction vessel at the same speed to carry out co-precipitation reaction;
[0020] S2. While step S1 is being performed, pump solution B into solution A at a constant speed, and dry to obtain a precursor after the reaction is terminated;
[0021] S3, mixing the precursor obtained in step S2 with a lithium salt, and sintering to obtain the positive electrode material of the lithium battery;
[0022] The solution A is a mixed solution containing water-soluble nickel salt, water-soluble manganese salt and water-soluble cobalt salt, wherein the molar ratio of Ni, Co, and Mn=(1-2x):x:x, where 0<x≤0.25 ;
[0023] The solution B is a mixed solution containing water-soluble nickel salt, water-soluble manganese salt and water-soluble cobalt salt, wherein the molar ratio of Ni, Co, and Mn=(1-2...
Embodiment 1
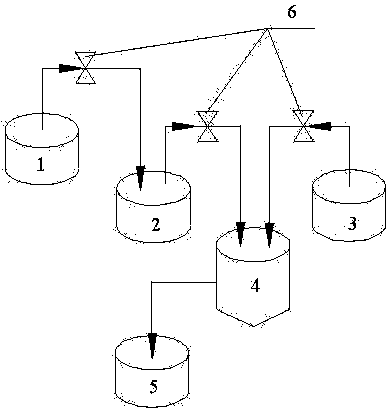
[0041] (1) if figure 1 As shown, the solution A1 of 2mol / L is charged in the second container 2, and the solution A1 is an aqueous solution of nickel sulfate, manganese sulfate and manganese sulfate, and the molar ratio Ni:Co:Mn=8:1:1; A container 1 is filled with 2mol / L solution B1, the solution B1 is an aqueous solution of nickel sulfate, manganese sulfate and manganese sulfate, the molar ratio Ni:Co:Mn=4:3:3; the volume of solution A2 and solution B2 same. Into the third container 3, the alkali solution is loaded, the alkali solution is a mixed system of sodium hydroxide and ammonia water, the concentration of sodium hydroxide in the alkali solution is 2mol / L, and the concentration of ammonia water is 1mol / L.
[0042] (2) First, the solution A1 contained in the second container 2 and the alkali solution contained in the third container 3 are respectively pumped into the reaction container 4 at a constant speed by the pump 6 for reaction, and at the same time, the solution ...
Embodiment 2
[0045] The same steps as in Example 1 were used to prepare the lithium battery positive electrode material S20 of this example, except that:
[0046] In step (1), solution A2 is an aqueous solution of nickel nitrate, manganese nitrate and manganese nitrate, the molar ratio Ni:Co:Mn=5:2.5:2.5, the concentration of solution A2 is 3mol / L; solution B2 is nickel nitrate, nitric acid The aqueous solution of manganese and manganese nitrate, the molar ratio Ni:Co:Mn=1:4.5:4.5, the concentration of solution B2 is 0.6mol / L; the volume of solution A2 and solution B2 is the same.
[0047] Through the above steps, the co-precipitation precursor S2 of the present embodiment (its chemical formula is Ni 0.44 co 0.28 mn 0.28 (OH) 2 ) and lithium battery cathode material S20 (its chemical formula is LiNi 0.44 co 0.28 mn 0.28 o 2 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com