Ultra-high purity hexafluoroethane preparation method
A technology of pure hexafluoroethane and hexafluoroethane, which is applied in the field of preparation of ultra-high-purity hexafluoroethane, can solve problems such as being unsuitable for industrial production, and achieve the effects of short adsorption time, high adsorption efficiency and environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~6
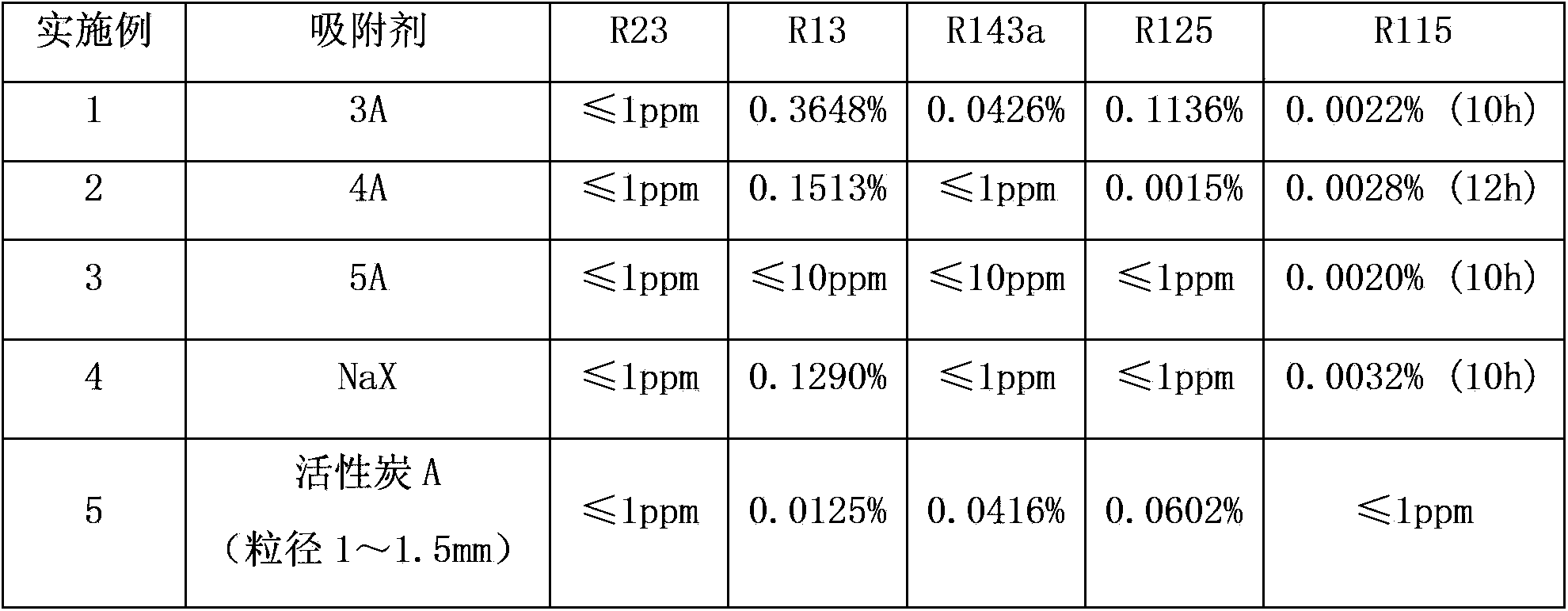
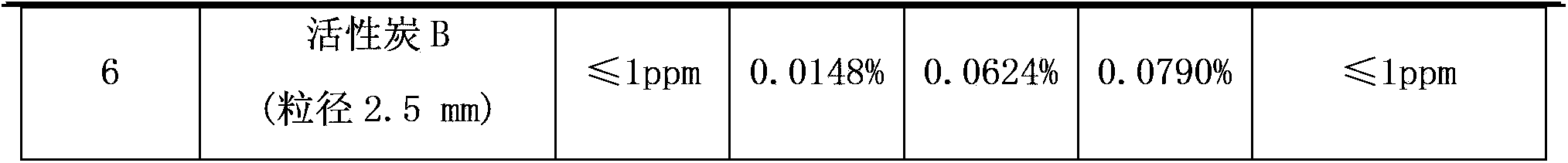
[0024] First put a certain amount of adsorbent into the muffle furnace with a tray, heat to 300°C for activation, keep the activation time for 3 hours and then take it out. Weigh 300g of the activated adsorbent and place it in a 1L stainless steel cylinder, seal it, and evacuate it, then add 400g of crude hexafluoroethane (containing R230.0137%, R130.4588%, R143a0.2378%, R1251 .5163, R1150.0058%), keep the temperature at -20°C, and start to adsorb until the adsorption equilibrium. The results after adsorption equilibrium are shown in Table 1. Except for special marks in Table 1, the rest are the data after adsorption for 5 hours.
[0025] Table 1
[0026]
[0027]
Embodiment 7~9
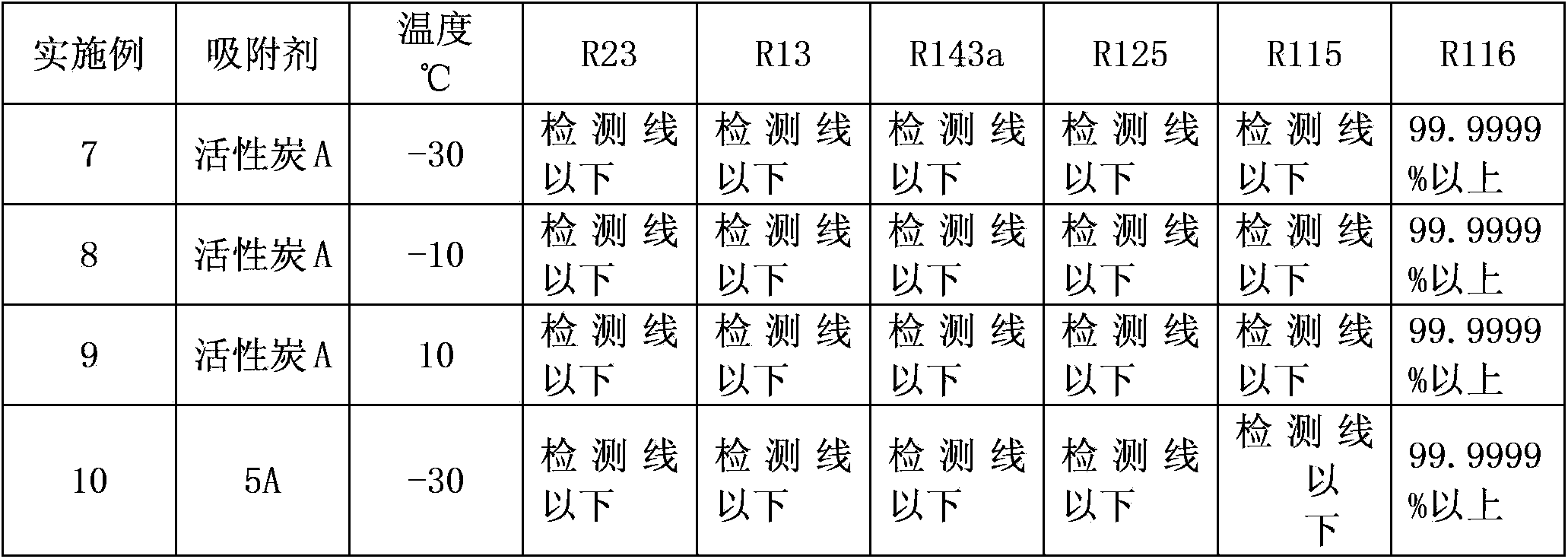
[0029] First put a certain amount of activated carbon A (particle size 1-1.5mm) into the oven with a tray, heat to 180°C for activation, and keep the activation time for 3 hours before taking it out. Weigh 130g of activated adsorbent activated carbon A and fill it in a stainless steel gas-solid adsorption column with an inner diameter of 25mm and a height of 90cm, seal and vacuumize. Then feed the crude hexafluoroethane, the impurity content of which is the same as in Example 1. At a temperature of -30, -10, 10°C and a pressure of 0.05-0.5 MPa (gauge pressure), the feed rate is 6.5 g / min from the top of the adsorption bed into the fixed adsorption bed. The gas after adsorption for 11 hours was condensed and collected, and the content of each component was analyzed by gas chromatography. The results are shown in Table 2.
Embodiment 10
[0031] First put a certain amount of 5A molecular sieve adsorbent into the muffle furnace with a tray, heat it to 300°C for activation, and keep the activation time for 3 hours before taking it out. Weigh 130 g of the activated adsorbent and fill it into a stainless steel gas-solid adsorption column with an inner diameter of 25 mm and a height of 90 cm, seal it, and evacuate it. Then feed the crude hexafluoroethane, the impurity content of which is the same as in Example 1. At a temperature of -30°C and a pressure of 0.05-0.5 MPa (gauge pressure), the feed rate is 4.0 g / min from the top of the adsorption bed into the fixed adsorption bed. The gas after adsorption for 8 hours was condensed and collected, and the content of each component was analyzed by gas chromatography. The results are shown in Table 2.
[0032] Table 2
[0033]
[0034] Embodiment 1-6 is intermittent adsorption, the 3A type molecular sieve of the present invention, 4A type molecular sieve, 5A type molecu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com