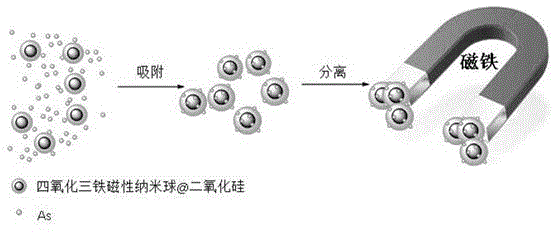
Fe3O4@SiO2 magnetic nanospheres as well as preparation method and application thereof
A magnetic nanosphere, magnetic technology, applied in chemical instruments and methods, other chemical processes, alkali metal oxides/hydroxides, etc., can solve the problems of small specific surface area of ferric oxide nanoparticles, and the adsorption effect needs to be improved. , to achieve the effect of low cost, large specific surface area and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 4
[0043] The preparation of embodiment 1 ferric oxide nanosphere
[0044] First, we used the co-precipitation method to prepare ferric oxide nanoparticles with a particle size of 8nm. The specific method is as follows:
[0045] FeCl 3 ·6H 2 O (0.4g, 1.4mmol) and FeSO 4 ·7H 2 O (0.2g, 0.7mmol), dissolved in 20mL of deionized water, ultrasonic 15min to remove oxygen.
[0046] The above solution was placed in a 50°C water bath under N 2 Under protection, after magnetic stirring for 15 min, 5 mL of ammonia water was slowly added dropwise (the dropping rate was 1 mL / min) therein, and the pH was adjusted to about 11, and the temperature was adjusted to 40°C.
[0047] Citric acid (0.16 g) was dissolved in 1 mL of water, added to the above reaction solution, and reacted for 1 h.
[0048] The resulting nanoparticles were prepared and washed twice with ethanol.
Embodiment 2 4
[0049] The preparation of embodiment 2 iron ferric oxide nanospheres
[0050] First, we used the co-precipitation method to prepare Fe3O4 nanoparticles with a particle size of 12nm. The specific method is as follows:
[0051] Will Fe 2 (SO 4 ) 3 (0.28g, 0.7mmol) and Fe(NO 3 ) 2 ·6H 2 O (0.20g, 0.7mmol), dissolved in 20mL of deionized water, ultrasonic 15min to remove oxygen.
[0052] The above solution was placed in a 60°C water bath under N 2 Under protection, after magnetic stirring for 60min, slowly dropwise (dropping speed is 1mL / min) NaHCO 3 Aqueous solution (0.02g / mL, 5mL), adjust the pH to around 10, and raise the temperature to 80°C.
[0053] Citric acid (0.16 g) was dissolved in 1 mL of water, added to the above reaction solution, and reacted for 1 h.
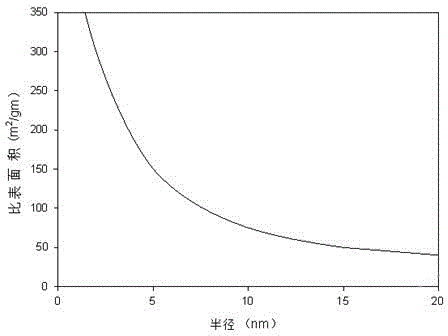
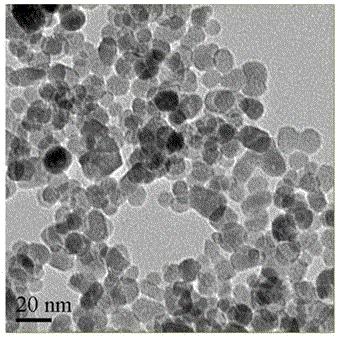
[0054] The resulting nanoparticles were prepared and washed twice with ethanol. Such as image 3 As shown, the average particle size of ferric oxide nanospheres prepared in this example is about 12 nm, and ...
Embodiment 3 4
[0055] The preparation of embodiment 3 iron ferric oxide nanospheres
[0056] First, we used the co-precipitation method to prepare Fe3O4 nanoparticles with a particle size of 12nm. The specific method is as follows:
[0057] FeCl 3 ·6H 2 O (0.4g, 1.4mmol) and Fe (NO 3 ) 2 ·6H 2 O (0.20g, 0.7mmol), dissolved in 20mL of deionized water, ultrasonic 15min to remove oxygen.
[0058] The above solution was placed in a 60°C water bath under N 2 Under protection, after magnetic stirring for 60min, slowly dropwise (dropping speed is 1mL / min) NaHCO 3 Aqueous solution (0.02g / mL, 5mL), adjust the pH to around 10, and raise the temperature to 80°C.
[0059] Citric acid (0.16 g) was dissolved in 1 mL of water, added to the above reaction solution, and reacted for 1 h.
[0060] The resulting nanoparticles were prepared and washed twice with ethanol.
[0061] with NaH 2 PO 4 solution or Na 2 HPO 4 solution instead of NaHCO 3 A solution can achieve the same effect.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com