Method for splitting DL-menthol by means of pre-column derivatization high performance liquid chromatography
A high-performance liquid chromatography and pre-column derivatization technology, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of low sensitivity and inability to achieve complete separation, and achieve the effect of accurate separation and separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] DL-menthyl benzoate used in each embodiment of the present invention, DL-menthyl p-nitrobenzoate, DL-menthyl o-chlorobenzoate, DL-menthyl p-methoxybenzoate, The synthetic method of p-phenylbenzoic acid DL-menthyl ester, 1-naphthoic acid DL-menthyl ester or cinnamic acid DL-menthyl ester, concrete steps are as follows:
[0042] In a 250mL three-necked flask, add the corresponding aromatic acid, DL-menthol and catalyst monohydrate p-toluenesulfonic acid in sequence, the molar ratio of which is 1:1.2:0.05, add 100mL of toluene as solvent, shake well and add the magnet, install the water separator Add toluene to the water separator, heat it at about 110°C, stir and reflux, condense and separate the water. After the reaction is completed, the water in the water separator will no longer increase, and the reaction system will be cooled to room temperature. Add 20 mL of water, separate the organic layer, wash the organic layer with saturated sodium bicarbonate and saturated bri...
Embodiment 1
[0045] Aromatic acids (benzoic acid, p-nitrobenzoic acid, o-chlorobenzoic acid, p-methoxybenzoic acid, p-phenylbenzoic acid, 1-naphthoic acid or cinnamic acid) (40.94 mmol), DL-menthol (7.68g, 49.13mmol) and catalyst monohydrate p-toluenesulfonic acid (0.39g, 2.05mmol), calculated by molar ratio, that is, aromatic acid: DL-menthol: catalyst monohydrate p-toluenesulfonic acid is 1 : 1.2: 0.05, then add 100mL of toluene as a solvent, shake well and add the magnet, install the water separator, spherical condenser and drying tube, fill the water separator with toluene, heat to about 110°C, stir and reflux, Condensate and separate the water. After the reaction is completed, that is, until the water in the water separator no longer increases, the reaction system is cooled to room temperature, 20mL of water is added, and the organic layer is separated. The organic layer is washed with saturated sodium bicarbonate and saturated brine successively, and controlled Spin dry at 40-50°C to...
Embodiment 2
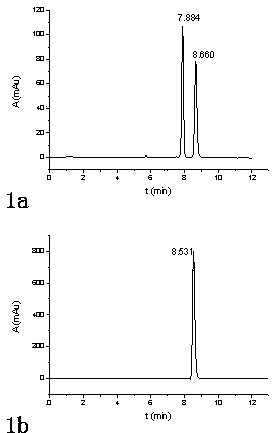
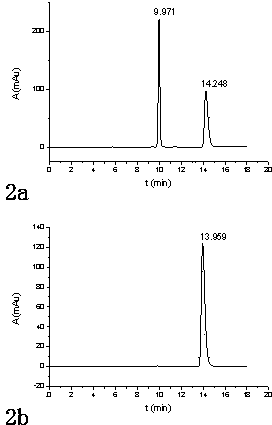
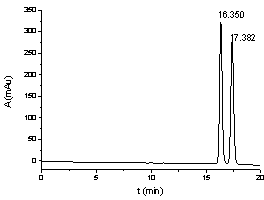
[0086] Adopt a kind of method of the present invention that utilizes pre-column derivatization high performance liquid chromatography to resolve DL-menthol, for 3 kinds of DL-menthyl benzoate esters and DL-menthyl p-methoxybenzoate obtained in Example 1 Ester and DL-menthyl cinnamate carry out high performance liquid chromatography separation, specifically comprise the steps:
[0087] (1) Take 25 mg of DL-menthyl benzoate, DL-menthyl p-methoxybenzoate and DL-menthyl cinnamate respectively, and mix them with acetonitrile and water. After the solution is dissolved, it is transferred to a 25mL volumetric flask, diluted to the mark with acetonitrile, shaken up, and used as the test solution;
[0088] Similarly, get 25 mg of corresponding standard L-menthol ester, dissolve and configure as the reference substance solution for testing in the same way as above;
[0089] (2) Inject the test solution and the reference solution respectively, carry out HPLC analysis according to the fol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com