Synthesis method of dithiazole quaternary ammonium salt
A synthesis method and technology of quaternary ammonium salts, applied in the direction of organic chemistry and the like, can solve problems such as difficulty in obtaining high-purity products, inability to obtain expected products, complex product components, etc., and achieve easy large-scale industrial production and application, easy operation, Promising effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
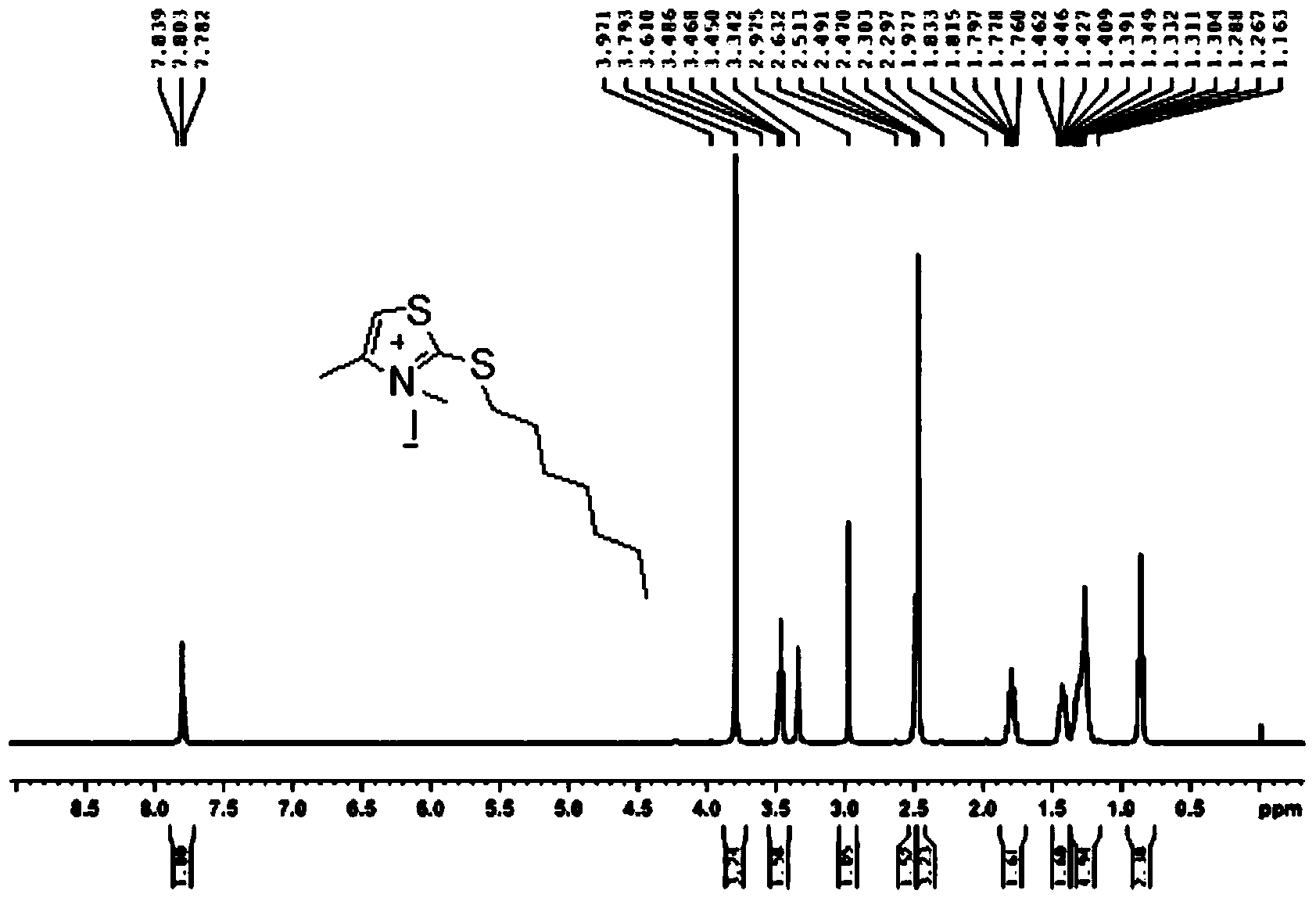
[0040] Synthesis of 3,4-Dimethyl-2-heptylthiothiazol-3-ium Iodide Salt
[0041]
[0042] 3,4-Dimethylthiazole-2(3H)-thioketone (reference: Acta Chemica Scandinavica, 1999, 53(10), 861–866) (2.42g, 16.8mmol) was added to a one-necked vial 1. Iodo-n-heptane (3.8g, 16.8mmol), heated to 120°C for 1h under the protection of nitrogen, a red viscous substance was formed, cooled, added ethanol and stirred quickly, a large amount of yellow solid was formed, filtered to obtain 3.66g , yield 58.7%.
[0043] h 1 NMR (400MHz, d 6 -DMSO): δH7.84(s,1H),3.79(s,3H),3.47(t,2H,J=7.2Hz),2.47(s,3H,CH 3 ),1.76-1.83(m,2H),1.39-1.46(m,2H),1.27-1.35(m,6H),0.85(t,3H,J=6.8Hz) (see figure 1 )
[0044] LC - MS: M + =244.0
Embodiment 2
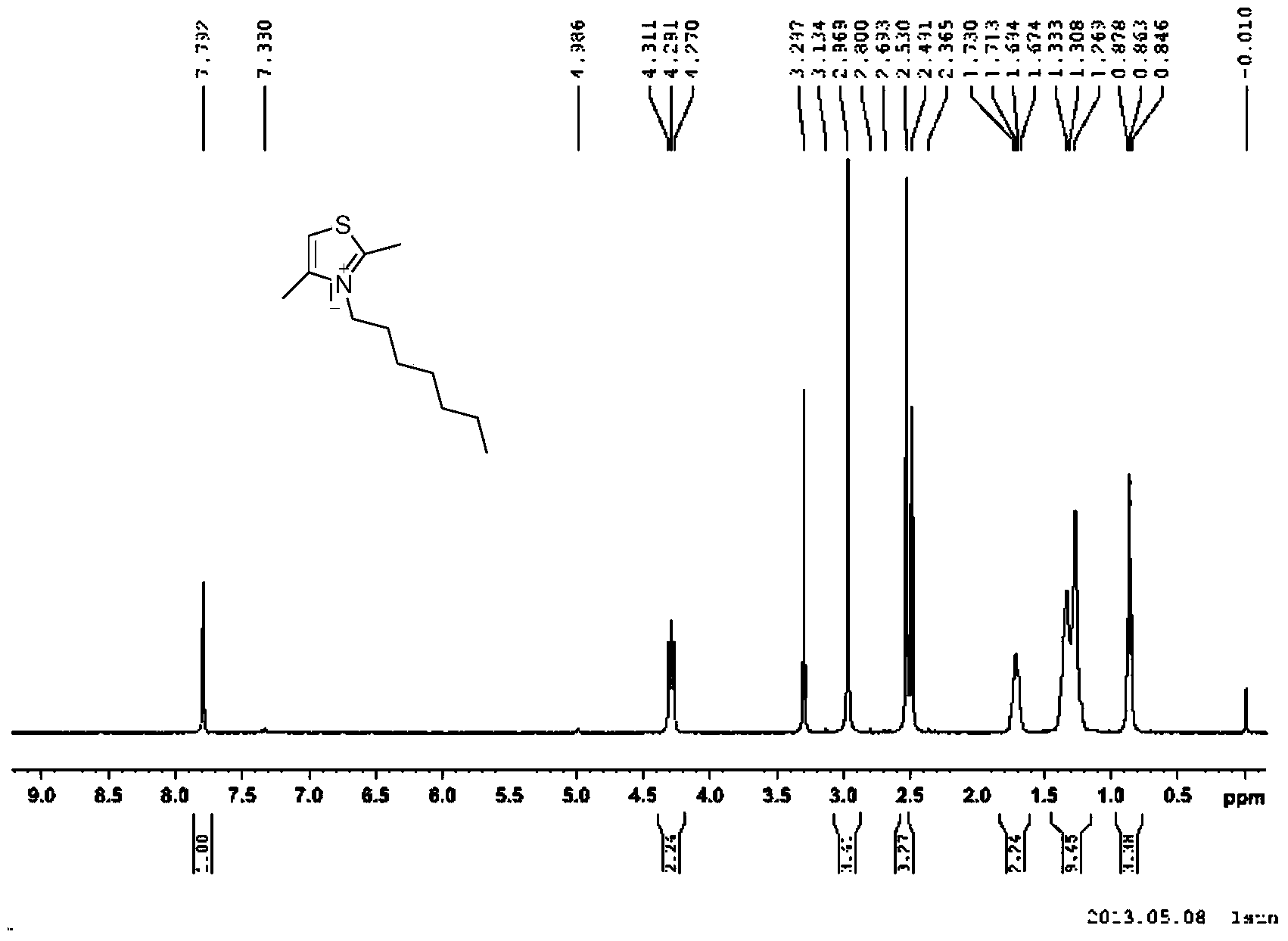
[0046]Synthesis of 2,4-Dimethyl-3-heptylthiazol-3-ium Iodide Salt
[0047]
[0048] Add 2,4-dimethylthiazole (21.5g, 0.19mmol), iodoheptane (81.2g, 0.57mmol) and 200ml of toluene into a single-necked bottle, heat to 120°C under nitrogen protection and reflux for 2 hours, then cool , The precipitated solid was washed with ethanol to obtain 46.0 g of white solid with a yield of 71.4%.
[0049] h 1 NMR (400MHz, d 6 -DMSO): δ7.79(s,1H),4.29(t,2H,J=8.0Hz),2.97(s,3H,CH 3 ),2.53(s,3H,CH 3 ),1.67-1.73(m,2H),1.327-1.33(m,8H),0.86(t,3H,J=6.8Hz) (see figure 2 )
[0050] LC - MS: M + =212.1
Embodiment 3
[0052] Synthesis of 2,4-Dimethyl-3-heptylthiazol-3-ium Iodide Salt
[0053] Put a magnet in a 250ml single-mouth bottle, add 2,4-dimethylthiazole (1.7g, 15mmol), iodo-n-heptane (8.48g, 37.5mmol), under nitrogen protection conditions, at 130°C Reaction, reacted for 16 hours, the reaction solution was separated, the lower layer was black oil, after cooling, fully stirred or sonicated, a large amount of solids were produced, added ethanol and sonicated or stirred to fully disperse the solid substances, and suction filtered to obtain 3.82g of white solids, with a yield of 75.0% .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com