Method for synthesizing dinucleoside diphosphate and dinucleoside triphosphote
A technology of dinucleoside diphosphate and nucleoside phosphoryl, which is applied in the field of chemical preparation of natural product biochemical reagents, can solve the problems of low reaction yield, difficult separation of by-products, and the need for protection of nucleosides, and achieve easy-to-obtain raw materials , easy product, fast coupling reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
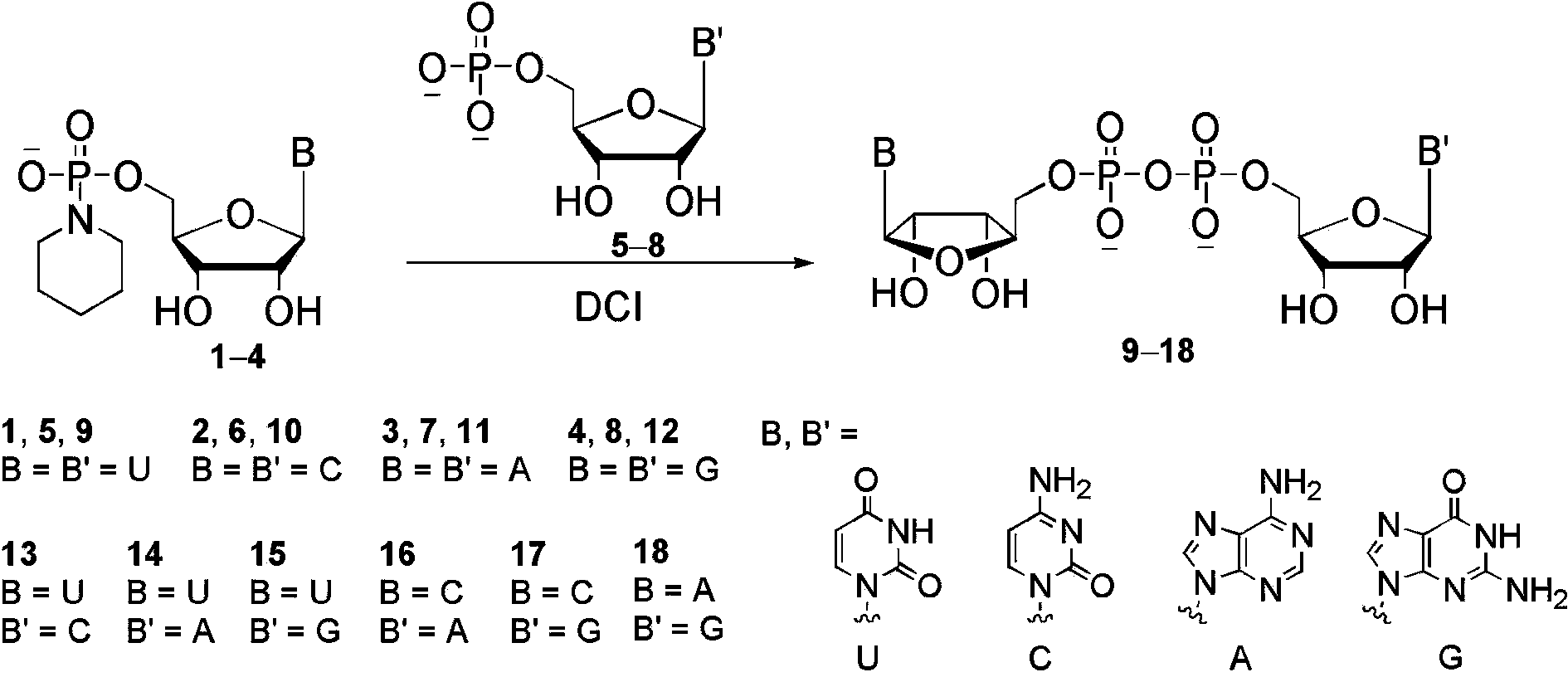
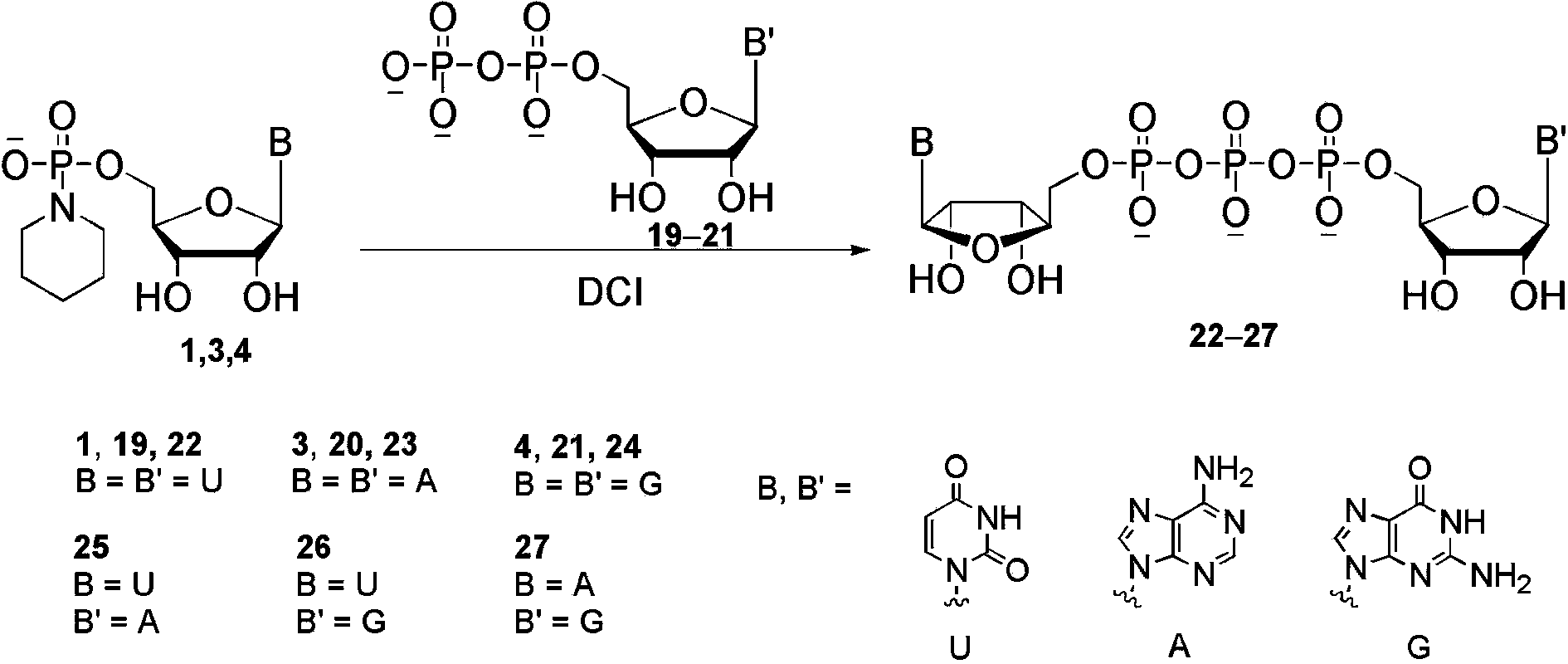
[0013] P 1 , P 2 - Diadenosine-5′,5′-diphosphate disodium salt ( 11 ) Synthesis: adenosine phosphoryl piperidine triethylamine salt ( 3 ,102 mg, 0.2 mmol), adenosine monophosphate triethylamine salt ( 7 , 143 mg, 0.32 mmol) and 4,5-dicyanoimidazole (85 mg, 0.72 mmol) were vacuum-dried in a 25 mL round bottom flask for 2 hours. Under argon protection, 3.0 mL of dry DMF was added and reacted at 20 °C for 16 hours. The reaction solution was concentrated under reduced pressure, and the crude product was obtained after adding sodium acetate aqueous solution (3.0 M, 1.0 mL) and 30 mL ethanol solution for precipitation and centrifugation. After dissolving in deionized water, Sephadex (DEAE-A25) ion-exchange column chromatography to obtain the product triethylamine salt, and then obtained after passing through sodium-type 732 cation-exchange resin P 1 , P 2 - Diadenosine-5′,5′-diphosphate disodium salt 118 mg white solid, yield 82%.
Embodiment 2
[0015] P 1 -Uridine-5′- P 2 - Cytidine-5′-diphosphate disodium salt ( 13 ) Synthesis: Cytidine phosphoryl piperidine triethylamine salt ( 2 , 98 mg, 0.2 mmol), uridine monophosphate triethylamine salt ( 5 , 144 mg, 0.34 mmol) and 4,5-dicyanoimidazole (87 mg, 0.74 mmol) were vacuum-dried in a 25 mL round bottom flask for 2 hours. Under the protection of argon, 3.0 mL of dry DMF was added and reacted at 20 °C for 16 hours. The reaction solution was concentrated under reduced pressure, and the crude product was obtained after adding sodium acetate aqueous solution (3.0 M, 1.0 mL) and 30 mL ethanol solution for precipitation and centrifugation. After dissolving in deionized water, Sephadex (DEAE-A25) ion-exchange column chromatography to obtain the product triethylamine salt, and then obtained after passing through sodium-type 732 cation-exchange resin P 1 -Uridine-5′- P 2 - Cytidine-5′-diphosphate disodium salt 100 mg, yield 74% as white solid.
Embodiment 3
[0017] P 1 -Adenosine-5′- P 2 - Guanosine-5′-diphosphate disodium salt ( 18 ) Synthesis: the guanosine phosphoryl piperidine triethylamine salt ( 4 , 106 mg, 0.2 mmol), adenosine monophosphate triethylamine salt ( 7 , 161 mg, 0.36 mmol) and 4,5-dicyanoimidazole (90 mg, 0.76 mmol) were vacuum-dried in a 25 mL round bottom flask for 2 hours. Under argon protection, 3.0 mL of dry NMP was added and reacted at 40 °C for 6 hours. Add 30 mL of ether solution to precipitate and centrifuge to obtain the crude product. After dissolving in deionized water, Sephadex (DEAE-A25) ion-exchange column chromatography to obtain the product triethylamine salt, and then obtained after passing through sodium-type 732 cation-exchange resin P 1 -Adenosine-5′- P 2 - Guanosine-5′-diphosphate disodium salt 103 mg, yield 70% as white solid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com