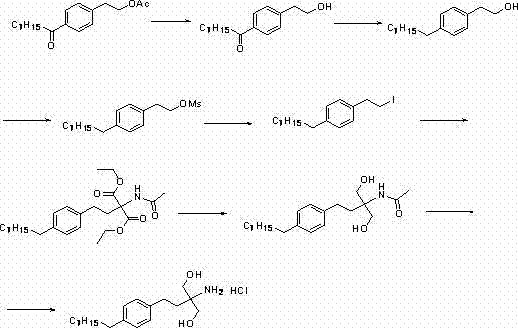
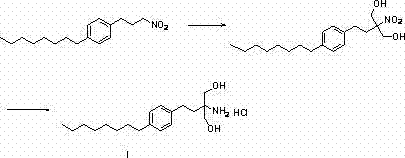
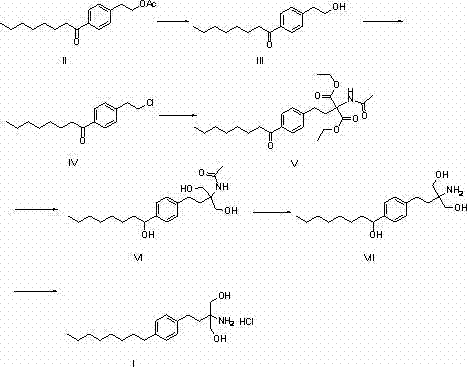
Method for preparing fingolimod hydrochloride
A technology of fingolimod hydrochloride and preparation process, which is applied in the field of pharmaceutical synthesis, can solve the problems of long reaction time, low yield, unsuitability for large-scale production, etc. High efficiency and easy post-reaction treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Preparation of compound (III). Add 200g of raw material compound (II), 800ml of absolute ethanol, 160g of sodium ethoxide into a 2L flask, install a thermometer and a reflux condensing device, stir and heat to 50-55°C, stir and react for 5 hours, TLC detects that the reaction is complete, cool, Pour the reaction solution into 1L saturated sodium chloride solution, add 800ml ethyl acetate, separate the layers, and evaporate the organic phase to dryness under reduced pressure to obtain 160g of crude product compound (III) as light yellow oil, which was directly carried out without further purification One step reaction.
Embodiment 2
[0042] Preparation of compound (III). Add 100g of raw material compound (II), 600ml of anhydrous methanol, and 130g of sodium methoxide into a 2L flask, install a thermometer and a reflux condenser, stir and heat to 60-65°C, stir and react for 8 hours, TLC detects that the reaction is complete, cool, Pour the reaction solution into 500mL saturated sodium chloride solution, add 400ml ethyl acetate, separate the layers, and evaporate the organic phase to dryness under reduced pressure to obtain 76g of the crude product as light yellow oil compound (III). The crude product was directly carried out without further purification. One step reaction.
Embodiment 3
[0044] Preparation of compound (IV). Add 160g of crude compound (III) and 900ml of dichloromethane to a 2L flask, install a thermometer and a reflux condensing device, add 220g of thionyl chloride in batches, after the addition is complete, stir and heat to 80-85°C, and stir for 2 hours. TLC detected that the reaction was complete, cooled, poured the reaction solution into 2L saturated ammonium chloride solution, stirred and separated the liquids, and evaporated the organic phase to dryness under reduced pressure to obtain 190 g of crude yellow oil compound (IV), which was directly processed without further purification Next reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com