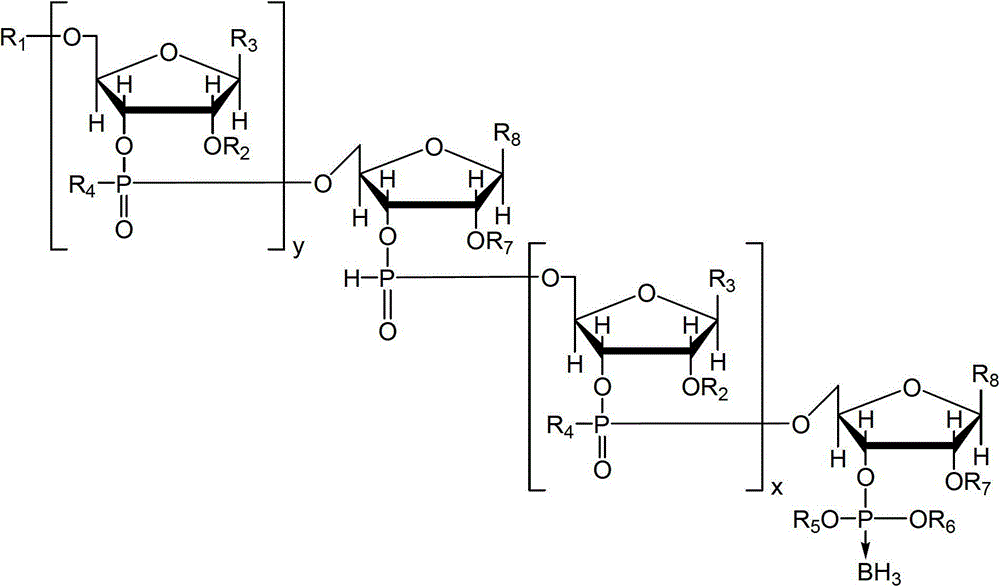
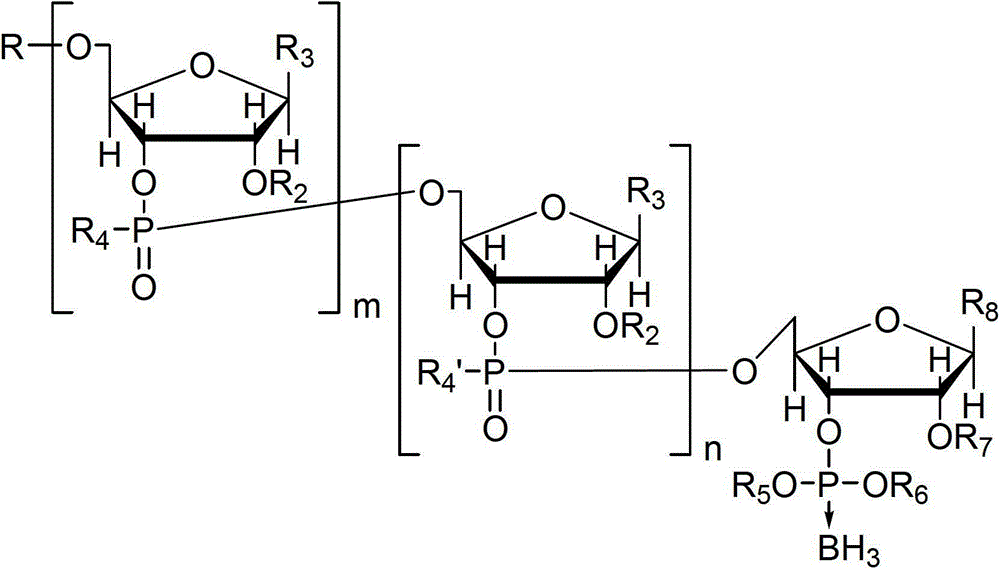
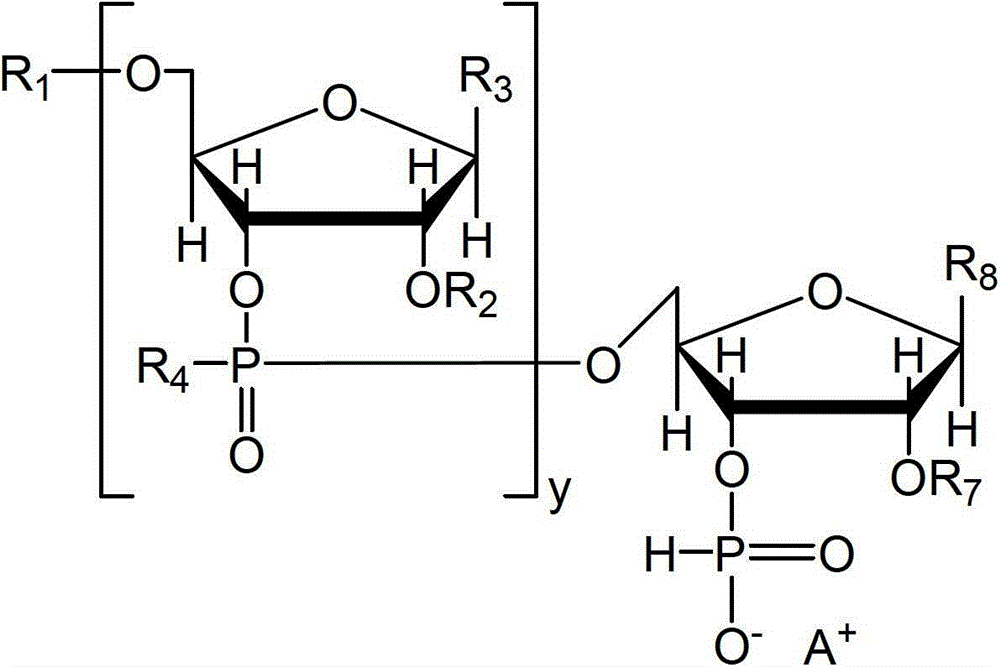
Nucleotide and/or oligonucleotide and preparation method thereof
An oligonucleotide and nucleotide technology, applied in chemical instruments and methods, preparation of sugar derivatives, sugar derivatives, etc., can solve the problems of high cost and small synthesis scale, and achieve the effect of saving raw materials and reducing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0062] According to a preferred embodiment of the present invention, wherein, the method may also include the following steps:
[0063] In the second liquid reaction medium, the substance of formula (4) is contacted with the substance of formula (5) to obtain the substance of formula (6);
[0064] Formula (5)
[0065]
[0066] Formula (6)
[0067] Among them, in formula (5) and formula (6), R 1 , R 2 , R 3 , R 4 , R 5 , R 6 , R 7 and R 8 The definition of is the same as that in formula (1).
[0068] Wherein, relative to 1 mole of the substance of the formula (4), the amount of the second liquid reaction medium can be 5-50 L, and the amount of the substance of the formula (5) can be 1-10 moles; the reaction temperature can be minus 50 ° C to 50°C; the reaction time can be 0.2-10 hours.
[0069] Wherein, the second liquid reaction medium may be pyridine or a mixed solution of pyridine and one or more of dichloromethane, acetonitrile, dioxane and tetrahydrofuran. ...
Embodiment 1
[0115] This embodiment synthesizes the substance of formula (1), wherein m and n are 0, R is trityl, R 5 and R 6 for -CH 2 CH 2 CN, R 7 is tert-butyldimethylsilyl, R 8 For uracil, the substance of the formula (1) is named as Tr[U]BH 3 .
[0116] PCl 3 (468mg, 3.4mmol) was dissolved in 20ml of the seventh liquid reaction medium (tetrahydrofuran), and at -78°C, added 2,6-lutidine (1.64g, 15.3mmol) and dissolved in 20ml of the seventh liquid reaction medium Formula (9) compound (wherein n is 0, R 1 is trityl, R 7 is tert-butyldimethylsilyl and R 8 Uracil, that is, 5'-O-Tr-2'O-TBDMS-rU, purchased from Shanghai Gemma Pharmaceutical Technology Co., Ltd., 1.0g, 1.7mmol), maintained for 5min.
[0117] Then, 3-hydroxypropionitrile (905 mg, 12.8 mmol) was added dropwise for 20 min.
[0118] Then, the temperature was raised to -20°C, and BH dissolved in the seventh liquid reaction medium (tetrahydrofuran, 7ml) was added dropwise. 3 (7 mmol), after maintaining at 0°C for 30 mi...
Embodiment 2
[0121] This embodiment synthesizes the substance of formula (3), wherein, y is 0, R 1 is trityl, R 7 is tert-butyldimethylsilyl, R 8 For uracil, the substance of the formula (1) is named Tr[U]PH, specifically through the above-mentioned Tr[U]BH 3 take off R 5 、BH 3 group and R 6 to fulfill.
[0122] At room temperature, in the third liquid reaction medium (CH 3 CN, 30ml), the Tr[U]BH 3 (784mg, 1mmol) was kept in contact with triethylamine (10ml) for 15min. Then concentrated under reduced pressure and sucked dry, promptly took off Tr[U]BH 3 R in 5 (-CH 2 CH 2 CN).
[0123] At 0°C, in the fourth liquid reaction medium (dichloromethane, 170ml), the dried solid product was kept in contact with dichloroacetic acid (5mmol) and methoxytrityl alcohol (735mg, 2.1mmol) for 10min . The reaction was quenched by adding 100 ml of saturated sodium bicarbonate solution. The reaction liquid was extracted twice with dichloromethane, 50 ml each time. Combine the extracted organic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com