A Simple, Rapid and Accurate Method for Determining Silver in Lead Anode Slime
An accurate measurement and lead anode slime technology, applied in the direction of weighing by removing certain components, can solve the problem that the addition amount and addition method of the precipitant hydrochloric acid solution cannot be well controlled, and the precipitation of silver chloride and silver ions cannot be precipitated. Complete, complete precipitation of silver chloride precipitates and other issues to achieve the effect of avoiding the problem of filtration, less reagent types and dosage, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
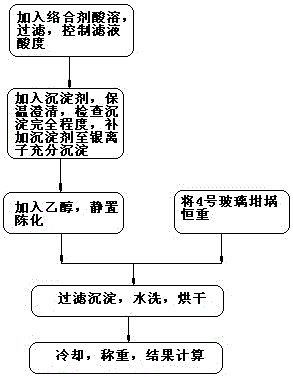
Image
Examples
Embodiment 1
[0047] This implementation example illustrates the determination result of this method to the lower content of silver in lead anode slime.
[0048] The solution preparation is completely operated according to the content of the invention (1).
[0049] Sample analysis: Weigh 2.0 grams of lead anode slime (main components: copper 8.06%, lead 20.16%, bismuth 5.71%, antimony 29.28%, arsenic 2.55%, selenium 1.03%, zinc 1.34%) samples in a series of 250mL beakers , add 20 mL of nitric acid and 2 g of tartaric acid, cover the watch glass, and heat on a low-temperature electric heating plate until the sample is completely dissolved. The following operations are completed according to the steps in the sample analysis (2) of the content of the invention. The obtained measurement results are shown in Table 1.
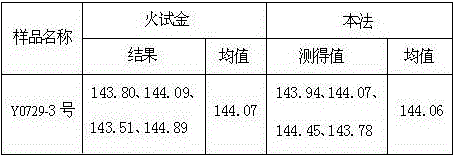
[0050] Table 1 Determination results of lower silver content in lead anode slime kg / t
[0051]
[0052] The significant difference (95% confidence level) for this method is: ...
Embodiment 2
[0058] This implementation example illustrates the determination results of this method for the medium content of silver in lead anode slime.
[0059] The solution preparation is completely operated according to the content of the invention (1).
[0060] Sample analysis: Weigh 2.0 grams of lead anode slime (main components: copper 10.16%, lead 21.11%, bismuth 7.51%, antimony 33.25%, arsenic 9.88%, selenium 1.27%, zinc 2.91%) samples in a series of 250mL beakers , add 20 mL of nitric acid and 2 g of tartaric acid, cover the watch glass, and heat on a low-temperature electric heating plate until the sample is completely dissolved. The following operations are completed according to the steps in the sample analysis (2) of the content of the invention. The obtained measurement results are shown in Table 2.
[0061] Table 2 Determination results of intermediate silver content in lead anode slime kg / t
[0062]
[0063] According to the requirements in the latest industry standa...
Embodiment 3
[0066] This implementation example illustrates the determination results of this method for high content silver in lead anode slime.
[0067] The solution preparation is completely operated according to the content of the invention (1).
[0068] Sample analysis: Weigh 2.0 grams of lead anode slime (main components: 13.38% copper, 21.9% lead, 8.98% bismuth, 37.43% antimony, 17.11% arsenic, 1.50% selenium, 4.5% zinc, 0.34% nickel, 0.38% cobalt ) sample in a series of 250mL beakers, add 20mL of nitric acid and 2 grams of tartaric acid, cover the watch glass, and heat on a low-temperature electric heating plate until the sample is completely dissolved. The following operations are completed according to the steps in the sample analysis (2) of the content of the invention. The obtained measurement results are shown in Table 3.
[0069] Table 3 Determination results of higher silver content in lead anode slime kg / t
[0070]
[0071] The significant difference (95% confidence le...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com