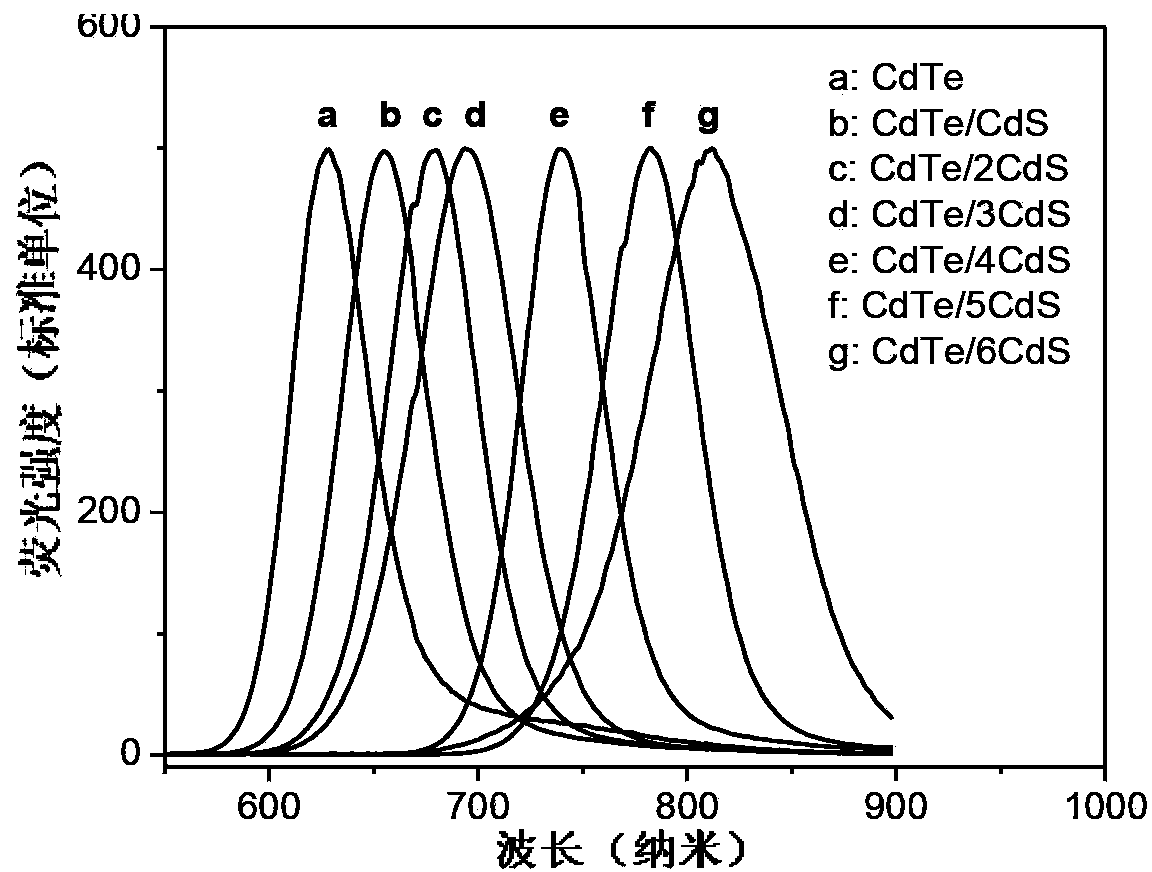
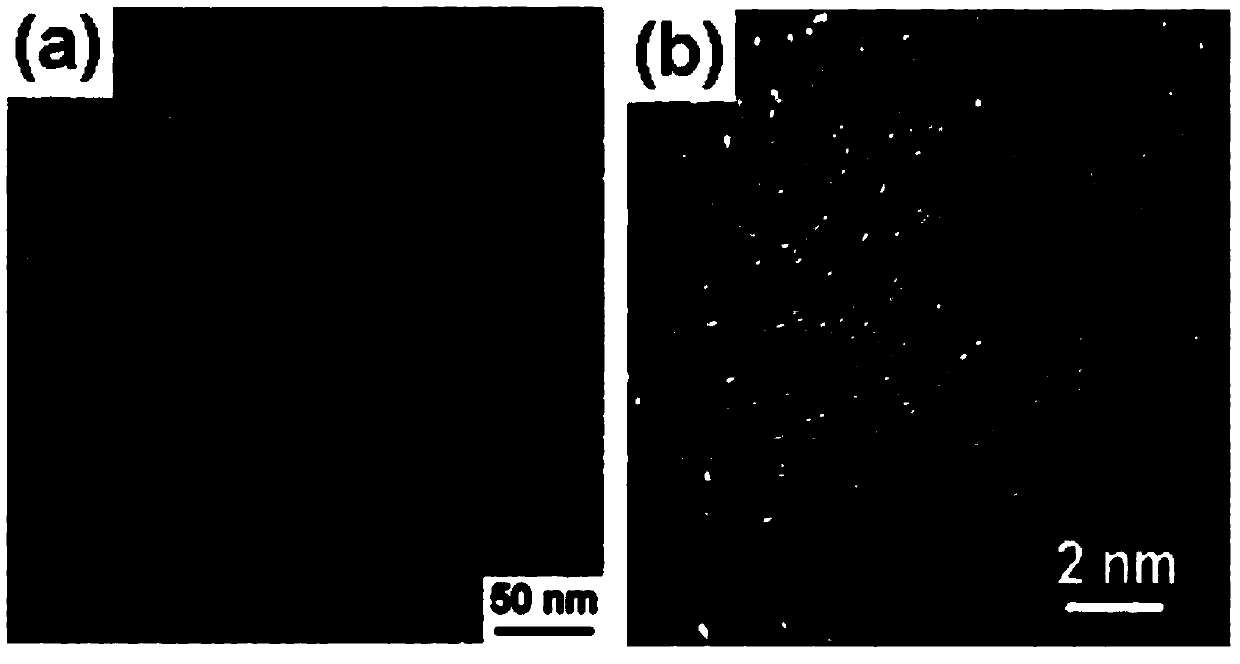
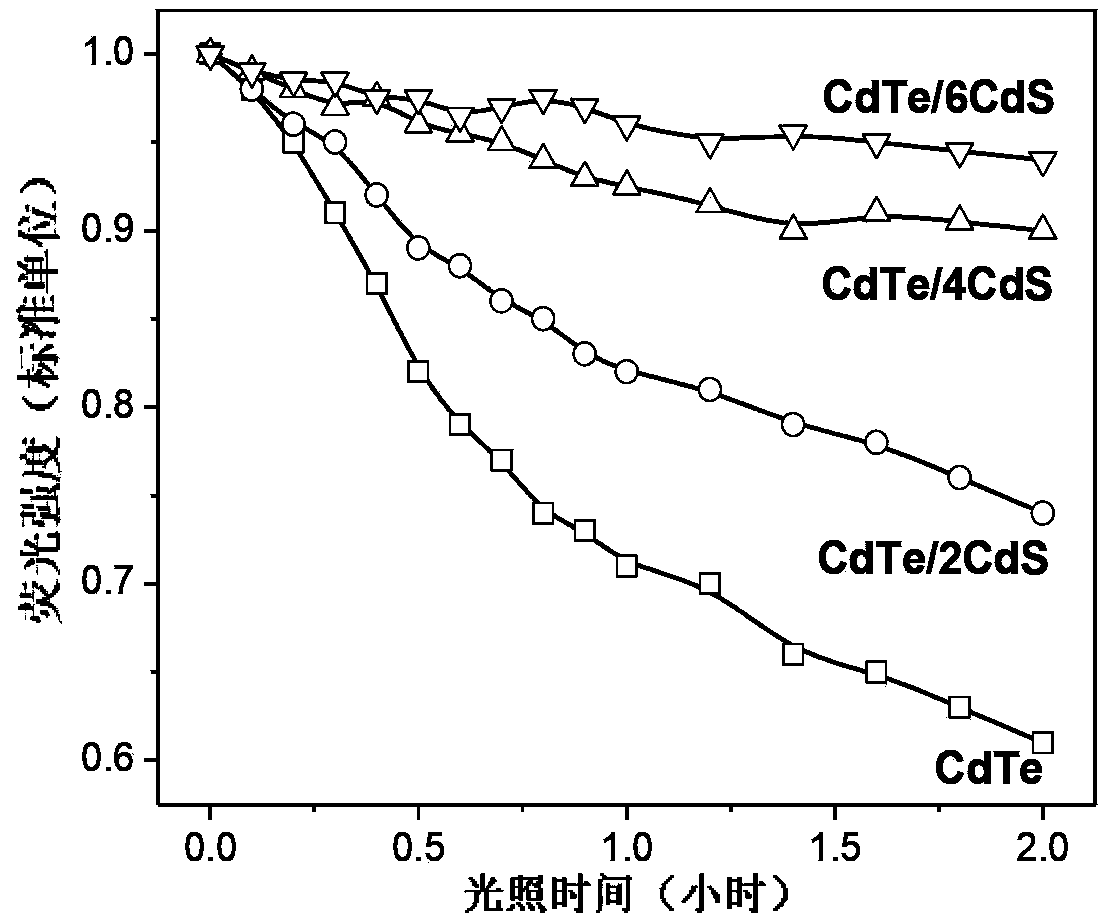
Near-infrared fluorescence emitting CdTe/CdS core/shell quantum dot, and preparation method thereof
A technology of fluorescence emission and quantum dots, which is applied in the field of near-infrared fluorescence emission quantum dots
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] in N 2 Under atmosphere and magnetic stirring, tellurium powder and sodium borohydride are added into the alkaline aqueous solution with a pH value of 9.0, and the molar ratio of the tellurium powder and sodium borohydride is 1:2. After reacting at 80° C. for 0.5 hour, a dark red clear liquid was obtained, which was the sodium telluride precursor solution.
[0056] According to the molar ratio of cadmium chloride, tellurium hydride precursor and glutathione is 1:5:10, the mixed aqueous solution of cadmium chloride and glutathione is added rapidly in the described sodium telluride hydride precursor solution, reacts Reflux for 4 hours to prepare a thiol-stabilized cadmium telluride quantum dot solution.
[0057] Utilize the empirical mathematical function of formula I~IX, calculate the concentration of the cadmium telluride quantum dot solution of gained to be 0.993×10 -6 mol / L, the quantum size of cadmium telluride is 3.221 nanometers, and the doses of cadmium chloride...
Embodiment 2
[0060] in N 2 Under atmosphere and magnetic stirring, tellurium powder and sodium borohydride are added into the alkaline aqueous solution with a pH value of 10.0, and the molar ratio of the tellurium powder and sodium borohydride is 1:2.5. After reacting at 70°C for 0.2 hours, a deep red clear liquid was obtained, which was the sodium telluride precursor solution.
[0061] According to the molar ratio of cadmium chloride, tellurium hydride precursor and glutathione being 1:4:8, the mixed aqueous solution of cadmium chloride and glutathione is quickly added to the sodium telluride hydride precursor solution, and the reaction Reflux for 5 hours to prepare a thiol-stabilized cadmium telluride quantum dot solution.
[0062] Utilize the empirical mathematical function of formula I~IX, calculate the concentration of the cadmium telluride quantum dot solution of gained to be 1.005×10 -6 mol / L, the size of cadmium telluride quantum is 3.204 nanometers, and the dosage of cadmium chl...
Embodiment 3
[0065] in N 2 Under atmosphere and magnetic stirring, tellurium powder and sodium borohydride are added into the alkaline aqueous solution with a pH value of 9.0, and the molar ratio of the tellurium powder and sodium borohydride is 1:2. After reacting at 80° C. for 0.5 hour, a dark red clear liquid was obtained, which was the sodium telluride precursor solution.
[0066] According to the molar ratio of cadmium chloride, tellurium hydride precursor and glutathione is 1:5:10, the mixed aqueous solution of cadmium chloride and glutathione is added rapidly in the described sodium telluride hydride precursor solution, reacts Reflux for 5 hours to prepare a thiol-stabilized cadmium telluride quantum dot solution.
[0067] Utilize the empirical mathematical function of formula I~IX, calculate the concentration of the cadmium telluride quantum dot solution of gained to be 1.047×10 -6 mol / L, the quantum size of cadmium telluride is 3.026 nanometers, and the doses of cadmium chloride...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap