Ophthalmic gel containing travoprost and timolol and preparation method thereof
A technology of travoprost and ophthalmic gel, which is applied in the field of pharmaceutical preparations and can solve problems such as insufficient elasticity, decreased comfort, blurred vision, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-7
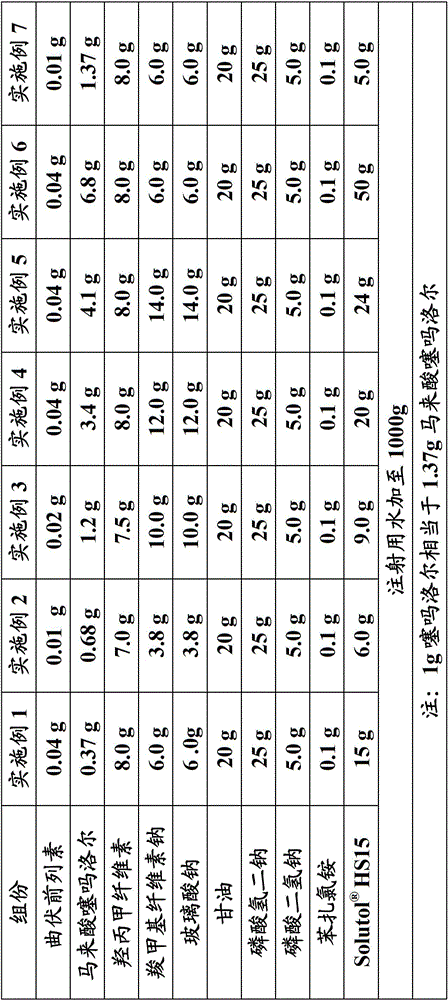
[0090] Embodiment 1-7: Preparation of ophthalmic gel 1-7
[0091] Prepare the ophthalmic gel of the present invention according to the corresponding prescription proportioning in Table 1.
[0092]Preparation method: (1) swell the prescription amount of hydroxypropyl methylcellulose, sodium carboxymethylcellulose and sodium hyaluronate with appropriate amount of water for injection, overnight, and set aside; (2) mix hypromellose matrix and carboxymethylcellulose The sodium matrix is fully mixed and set aside; (3) get the prescription amount of travoprost and place it in a clean beaker, add the prescription amount HS15 solution, stir until completely dissolved, and set aside; (4) Take about 500g of water for injection at 50-60°C, dissolve the prescribed amount of timolol maleate, glycerin and phosphate in turn, stir well, (5) Add the solution of (3) to solution (4), and rinse the beaker (3) with a small amount of water for injection, merge the cleaning liquid into the mix...
experiment example 1
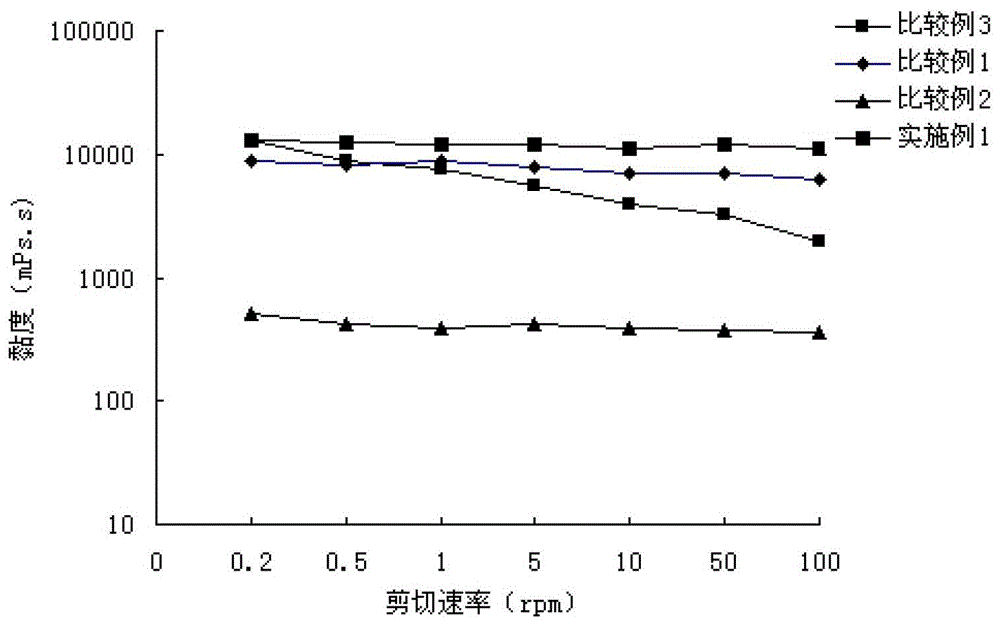
[0105] Experimental Example 1: Rheology Experiment
[0106] 1. Test drug
[0107] The ophthalmic gel samples prepared in Example 1 and Comparative Examples 1-3.
[0108] 2. Experimental method
[0109] The colloid properties and rheological properties of Example 1 and Comparative Example 1-3 are compared.
[0110] From the appearance of the preparation, the appearance of the gel preparation obtained in Example 1 is clear and transparent, and has suitable viscosity and elasticity. The preparations obtained in Comparative Example 1 and Comparative Example 3 had high viscosity and poor elasticity, and the preparations could not be dripped down. The preparation obtained in Comparative Example 2 has a clear appearance, but has a low viscosity.
[0111] The viscosity of Example 1 and Comparative Examples 1-3 was measured by rotational viscosity method (LVDV-II rotational viscometer 25°C 4# rotor).
[0112] 3. Experimental results
[0113] Such as figure 1 shown.
[0114] F...
experiment example 2
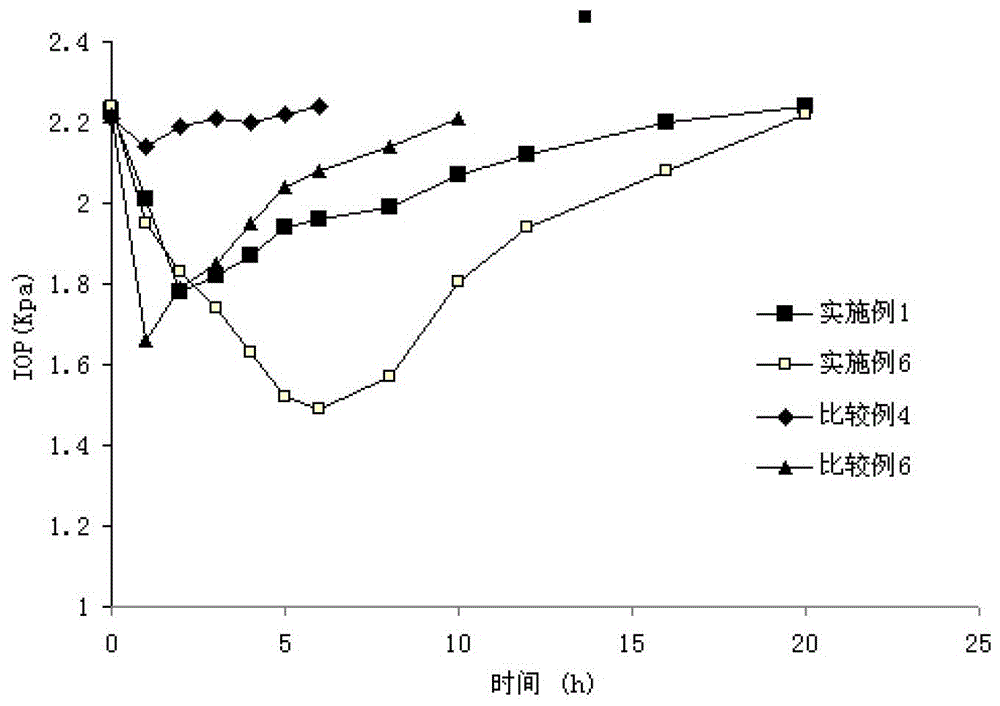
[0115] Experimental Example 2: Eye Irritation Test
[0116] 1. Purpose of the experiment:
[0117] Observe the irritation reaction of animals after eye administration of the ophthalmic gel of the present invention.
[0118] 2. Experimental animals:
[0119] New Zealand rabbits, weighing 2.0-2.5kg, male and female, provided by the Experimental Animal Center of Shenyang Pharmaceutical University. The test animals did not have any inflammatory reaction or eye damage.
[0120] 3. Test drugs:
[0121] The ophthalmic gel samples prepared in Example 1, Comparative Example 4 and Comparative Example 5.
[0122] 4. Experimental method:
[0123] Using the self-comparison method of the left and right sides of the same body, select 4 rabbits in each group, half male and half male, and check the eyes of each animal within 24 hours before the experiment. Animals with eye irritation symptoms, corneal defects and conjunctival damage cannot be used in the experiment. .
[0124] ⑴Single-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com