Xanthic acid acyl ester collecting agent and preparation and application methods thereof
A technology of acyl xanthate and its application method, which is applied in the field of acyl xanthate collectors, can solve the problems of no acyl xanthate, improve flotation grade and recovery rate, and realize industrial production and operation simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The synthesis of embodiment 1 isobutyl xanthate acetyl ester
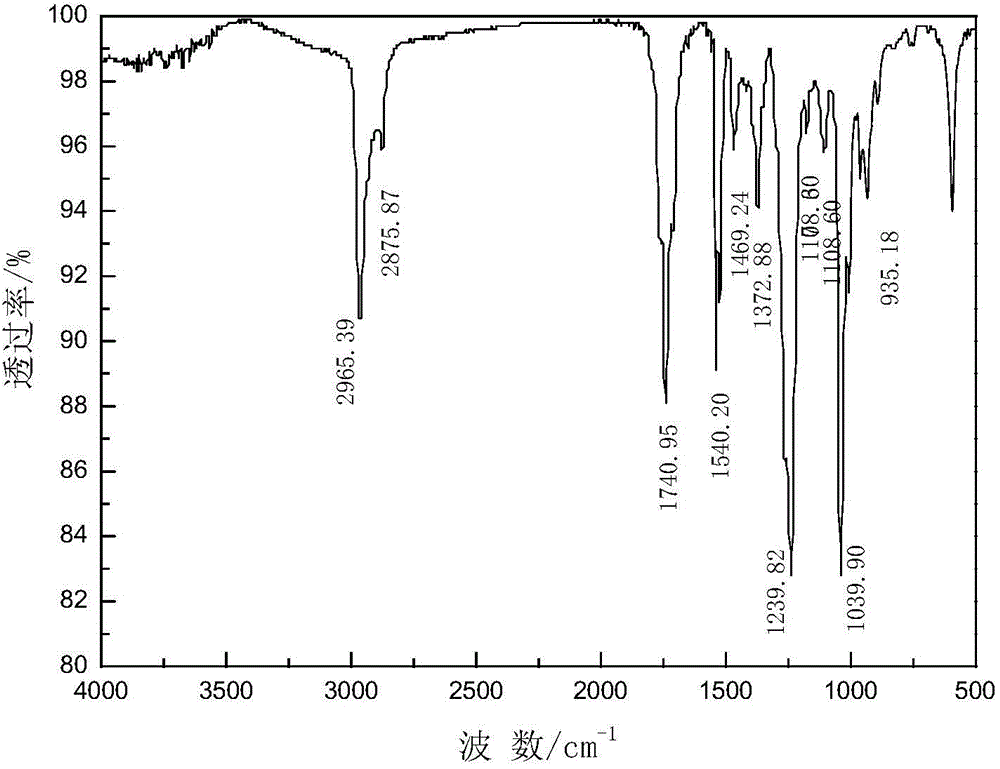
[0037] Add 15.70 parts of acetyl chloride and 32.68 parts of sodium isobutyl xanthate to 49 parts of solvent methylene chloride, stir and react at 20°C for 1.5 hours, remove sodium chloride by filtration, and obtain acetyl isobutyl xanthate and dichloromethane A mixed solution of methyl chloride, and then distilled under reduced pressure to remove the solvent to obtain the acetyl isobutyl xanthate product, the product yield based on acetyl chloride was 95.05%, and the purity was 84.56%. The product is characterized after being separated and purified by silica gel column chromatography. The infrared spectrum analysis and nuclear magnetic resonance analysis spectra of the product are shown in figure 1 with Figure 4 , see Table 1 and Table 2 for the data.
Embodiment 2
[0038] The synthesis of embodiment 2 isobutyl xanthate benzoyl esters
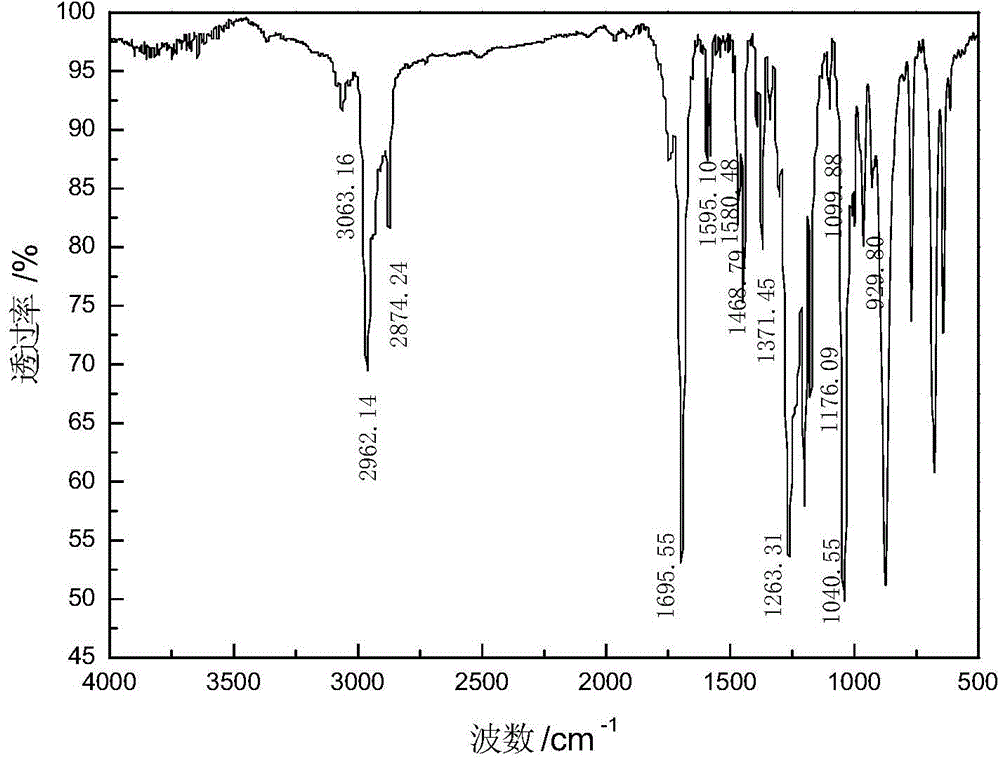
[0039] Add 28.11 parts of benzoyl chloride and 34.40 parts of sodium isobutyl xanthate to 64 parts of solvent methylene chloride, stir and react at 30°C for 2 hours, remove sodium chloride by filtration, and obtain benzoyl isobutyl xanthate and The mixed solution of dichloromethane is then distilled under reduced pressure to remove the solvent to obtain the isobutyl xanthate benzoyl ester product, the product yield based on benzoyl chloride is 97.40%, and the purity is 87.95%. The product is characterized after being separated and purified by silica gel column chromatography. The infrared spectrum analysis and nuclear magnetic resonance analysis spectra of the product are shown in figure 2 with Figure 5 , see Table 1 and Table 2 for the data.
Embodiment 3
[0040] The synthesis of embodiment 3 isobutyl xanthate octanoyl esters
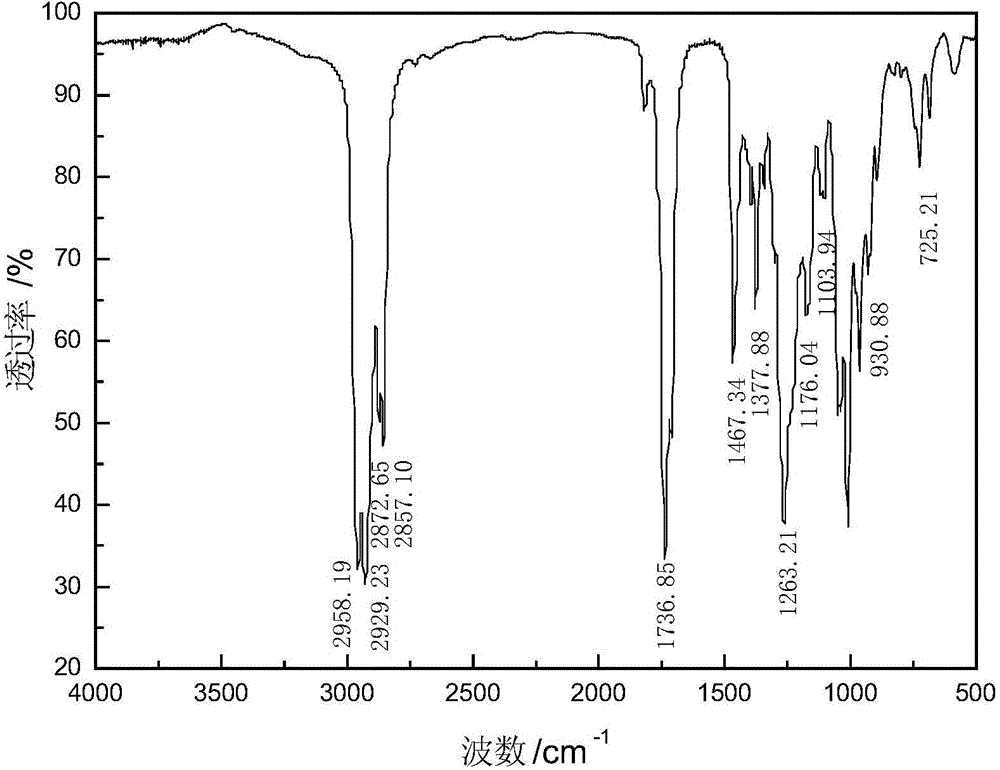
[0041] Add 32.53 parts of octanoyl chloride and 36.12 parts of sodium isobutyl xanthate to 69 parts of solvent dichloromethane, stir and react at 35°C for 2 hours, remove sodium chloride by filtration, and obtain octanoyl isobutyl xanthate and dichloromethane The mixed solution, and then the solvent was removed by distillation under reduced pressure to obtain the octanoyl isobutyl xanthate product, the product yield based on octanoyl chloride was 95.27%, and the purity was 86.42%. The product is characterized after being separated and purified by silica gel column chromatography. The infrared spectrum analysis and nuclear magnetic resonance analysis spectra of the product are shown in image 3 with Image 6 , see Table 1 and Table 2 for the data.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com