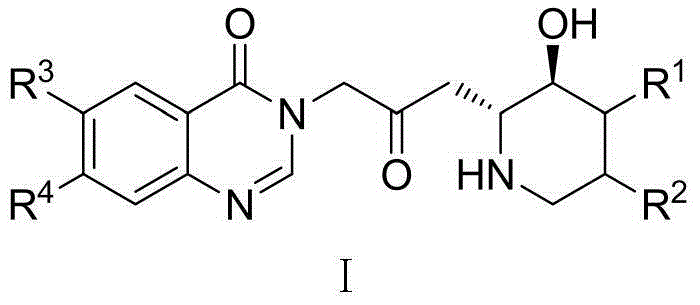
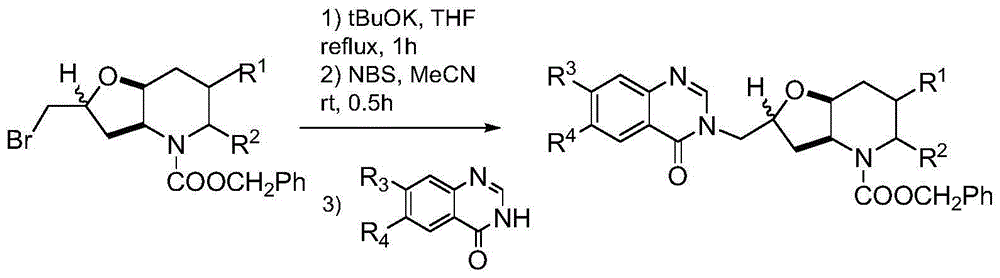
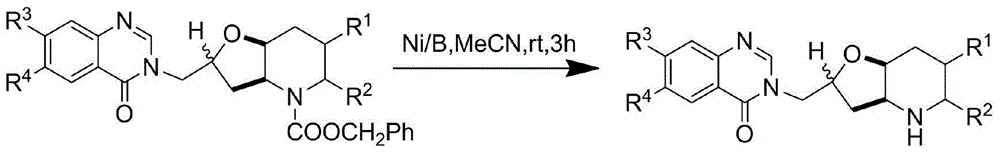
Orixine derivative and application of orixine derivative in preparation of medicine for resisting drug-fast bacteria
A technology of derivatives and persine, which is applied in the field of preparation of anti-drug-resistant bacteria drugs, can solve the problems of antibacterial drugs that have not been reported in the literature, and achieve a strong inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1: 3-[3-(3-Hydroxy-2-piperidinyl)-2-oxopropyl]-6-methoxy-7-(1-methylpiperidine-4-methoxy) Synthesis of -4(3H)-quinazolinone
[0033] Towards Potassium tert-butoxide (0.74g, 6.0mmol) was added to anhydrous tetrahydrofuran solution of (1.78g, 5.0mmol), and heated to reflux for 1h. After cooling, a mixture of NBS (1.07 g, 6.0 mmol) in acetonitrile and water (5 ml each) was added, and stirred at room temperature for 0.5 h. Add 100ml of sodium thiosulfate aqueous solution (mass fraction 10%), extract with 100ml of ethyl acetate, wash, dry, filter and concentrate with 6-methoxyl-7-(1-methylpiperidine-4-methyl Oxy)-4(3H)-quinazolinone (1.82g, 6.0mmol) and anhydrous potassium carbonate (0.83g, 6.0mmol) were added to anhydrous DMF (10ml), and stirred at room temperature for 1h. Saturated brine (100 ml) was added, followed by extraction twice with ethyl acetate (50 ml). The organic layer was washed with saturated brine (50ml), dried, filtered, concentrated and purifi...
Embodiment 2
[0036] Example 2: 7-benzyloxy-6-methoxy-3-[3-(3-hydroxyl-2-piperidinyl)-2-oxopropyl]-4(3H)-quinazolinone synthesis
[0037] Towards Potassium tert-butoxide (0.74g, 6.0mmol) was added to anhydrous tetrahydrofuran solution of (1.78g, 5.0mmol), and heated to reflux for 1h. After cooling, a mixture of NBS (1.07 g, 6.0 mmol) in acetonitrile and water (5 ml each) was added, and stirred at room temperature for 0.5 h. Add 100ml of sodium thiosulfate aqueous solution (mass fraction 10%), extract with 100ml of ethyl acetate, wash, dry, filter and concentrate with 7-benzyloxy-6-methoxyl-4(3H)-quinazole Linone (1.70g, 6.0mmol) and anhydrous potassium carbonate (0.83g, 6.0mmol) were added to anhydrous DMF (10ml), and stirred at room temperature for 1h. Saturated brine (100 ml) was added, followed by extraction twice with ethyl acetate (50 ml). The organic layer was washed with saturated brine (50ml), dried, filtered, concentrated and purified by column chromatography. The purified pro...
Embodiment 3
[0040] Example 3: 7-(3-diethylaminopropoxy)-6-methoxy-3-[3-(3-hydroxy-2-piperidinyl)-2-oxopropyl]–4(3H )-The synthetic method of quinazolinone
[0041] Towards Potassium tert-butoxide (0.74g, 6.0mmol) was added to anhydrous tetrahydrofuran solution of (1.78g, 5.0mmol), and heated to reflux for 1h. After cooling, a mixture of NBS (1.07 g, 6.0 mmol) in acetonitrile and water (5 ml each) was added, and stirred at room temperature for 0.5 h. Add 100ml of sodium thiosulfate aqueous solution (mass fraction 10%), extract with 100ml of ethyl acetate, wash, dry, filter and concentrate with 7-(3-dimethylaminopropoxy)-6-methoxy- 4(3H)-Quinazolinone (1.83g, 6.0mmol) and anhydrous potassium carbonate (0.83g, 6.0mmol) were added to anhydrous DMF (10ml), and stirred at room temperature for 1h. Saturated brine (100 ml) was added, followed by extraction twice with ethyl acetate (50 ml). The organic layer was washed with saturated brine (50ml), dried, filtered, concentrated and purified by...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com