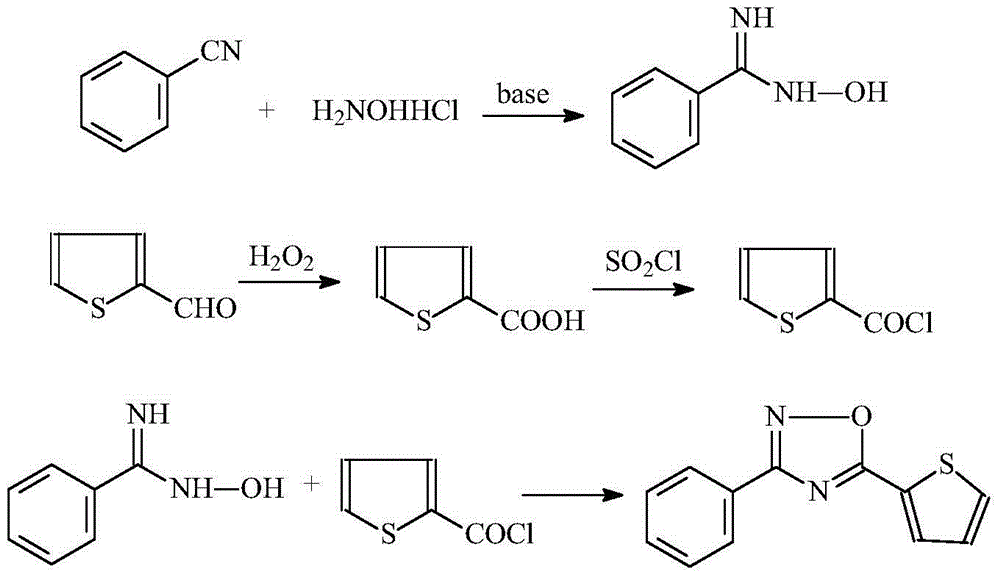
Synthetic technology for 3-phenyl-5-(thiophene-2-yl)-1,2,4-oxadiazole
A synthesis process and oxadiazole technology are applied in the field of pesticide technical preparation, can solve the problems of polluting the environment, do not involve the synthesis of the intermediate 2-thiophenecarbonyl chloride and the intermediate N-hydroxybenzamidine, etc., and improve the total yield. efficiency, avoiding manganese dioxide solids, shortening reaction steps and time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
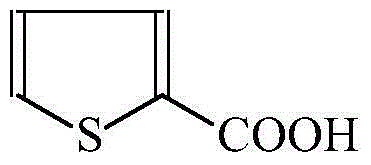
[0023] 1. Synthesis of 12-thiophenecarboxylic acid
[0024]
[0025] Add 16.81g (0.15mol) of 2-thiophenecarbaldehyde, 150ml of methanol, and 3.0g (0.028mol) of sodium chlorate into a 250ml four-necked reaction flask, cool to 10-15°C, and then dropwise add 30wt.% peroxide Hydrogen 6.12g (0.18mol), the dropwise addition temperature does not exceed 20°C, after the dropwise addition is completed, the room is raised to 70°C, and the temperature is continued for 10h; under the condition of 50°C, the methanol is distilled off under reduced pressure to obtain 18.2g of white solid, which is collected The rate is 89%.
[0026] 1.2 Synthesis of 2-thiophenoyl chloride
[0027]
[0028] Dissolve 44.5 g (0.347 mol) of 2-thiophenecarboxylic acid obtained in step 1) in 150 ml of dichloromethane solvent, add 0.5 ml of DMF, and add 120 ml of thionyl chloride (1.6 mol) dropwise under stirring. Complete, heat up and reflux for 12 hours, LC tracking (liquid chromatography) until the reacti...
Embodiment 2
[0036] The synthesis of 2-thiophenecarboxylic acid, the synthesis of 2-thiophenoyl chloride and the synthesis of N-hydroxybenzamidine are the same as in Example 1.
[0037] Synthesis of 3-phenyl-5-(thiophen-2-yl)-1,2,4-oxadiazole
[0038] In a 250ml four-necked reaction flask, add 10.88g (0.08mol) of N-hydroxybenzamidine and 80ml of acetone and stir to dissolve. At room temperature, slowly add 42.4g (0.16mol) of 40% sodium carbonate solution, and then dropwise add 11.72g (0.08mol) 2-thiophenoyl chloride, stirred at room temperature for 2 hours, then raised the temperature and refluxed to continue the reaction for 6 hours. After the reaction was completed, the reactant was poured into 100ml of water for layering, and the water layer was extracted with acetone. The acetone was evaporated under reduced pressure, and cooled to precipitate Solid, 14.7 g of white solid was obtained, the yield was 81.2%, and the melting point was 108-110°C.
Embodiment 3
[0040] The synthesis of 2-thiophenecarboxylic acid, the synthesis of 2-thiophenoyl chloride and the synthesis of N-hydroxybenzamidine are the same as in Example 1.
[0041] Synthesis of 3-phenyl-5-(thiophen-2-yl)-1,2,4-oxadiazole
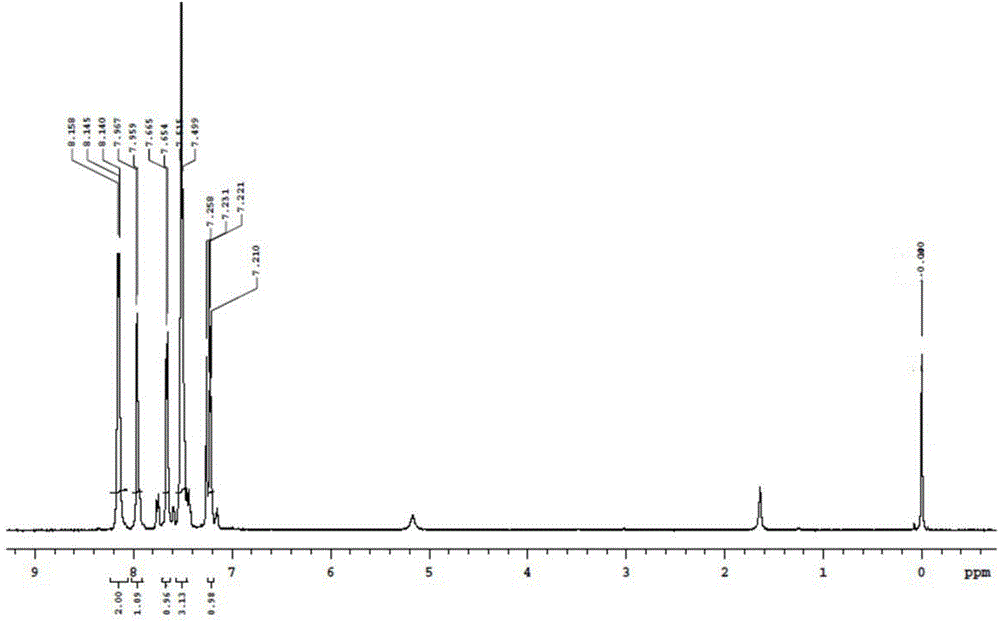
[0042] Add 10.88g (0.08mol) of N-hydroxybenzamidine and 80ml of tetrahydrofuran into a 250ml four-necked reaction flask and stir to dissolve. At room temperature, slowly add 55.2g (0.16mol) of 40% potassium carbonate solution, and then dropwise add 11.72g (0.08mol) 2-thiophenoyl chloride, stirred at room temperature for 2h, then raised the temperature and refluxed to continue the reaction for 6h, after the reaction was completed, the reactants were poured into 100ml of water for layering, the aqueous layer was extracted with toluene, and tetrahydrofuran and toluene were evaporated under reduced pressure. The solid was precipitated by cooling to obtain 15.3 g of a white solid with a yield of 86.3% and a melting point of 108-110° C. The nuclear magnet...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com