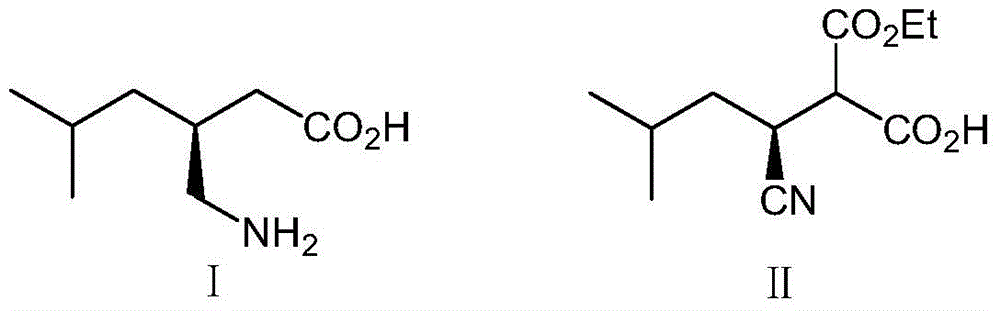Thermomyces lanuginosa lipase mutants, encoding genes and their applications
A thermophilic mycete and lipase technology, which is applied in application, genetic engineering, plant genetic improvement, etc., can solve the problems of harsh reaction conditions, large amount of toxic and harmful solvents, and lengthy process steps, etc., and achieve the effect of improving the vigor of parents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: Error-prone PCR (error-prone PCR) constructs lipase mutation library
[0028] The plasmid pET28-Lip-T cloned with the gene encoding Thermomyces lanuginosus DSM 10635 lipase Lip triple mutant Lip-T (S88T / A99N / V116D, amino acid sequence SEQ ID NO.1, nucleic acid sequence SEQ ID NO.2) was used as a template , using T7promoter primers and T7terminator primers (Table 1) for error-prone PCR amplification, random introduction of mutations. PCR system includes: Mg-free 2+ 1×Taq Buffer, 0.2mM dGTP, 0.2mM dATP, 1.0mM dCTP, 1.0mMdTTP, 7mM MgCl 2 , 0.2mM MnCl 2 , T7promoter primer and T7terminator primer 0.2μM each, plasmid pET28-Lip-T 50ng, Taq DNA polymerase 5U, water up to 100μl. PCR conditions were pre-denaturation at 94°C for 5 minutes, 30 cycles: 94°C for 30s, 55°C for 30s, 72°C for 1min, and extension at 72°C for 10min after the cycle. After the PCR was positive by 0.9% agarose gel electrophoresis analysis, 1 μl of the PCR solution was taken as a template f...
Embodiment 2
[0029] Example 2: Screening of lipase mutant library
[0030] The high-throughput screening technology for screening of mutant libraries is described in (Bioorgan. Med. Chem., 1999, 7: 2183-2188). The specific process is as follows:
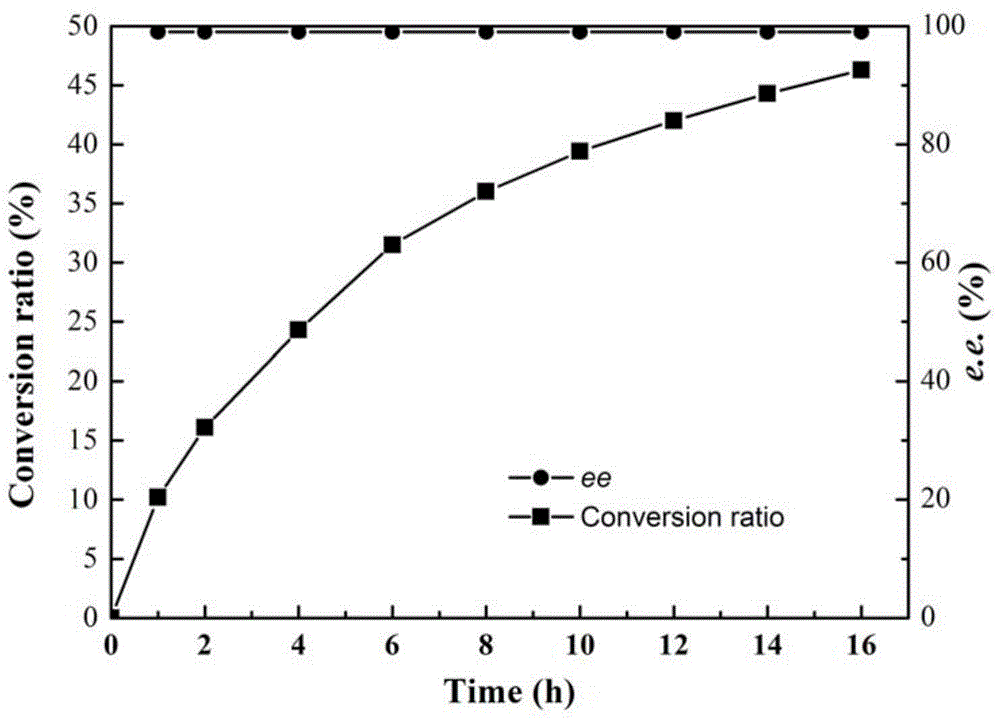
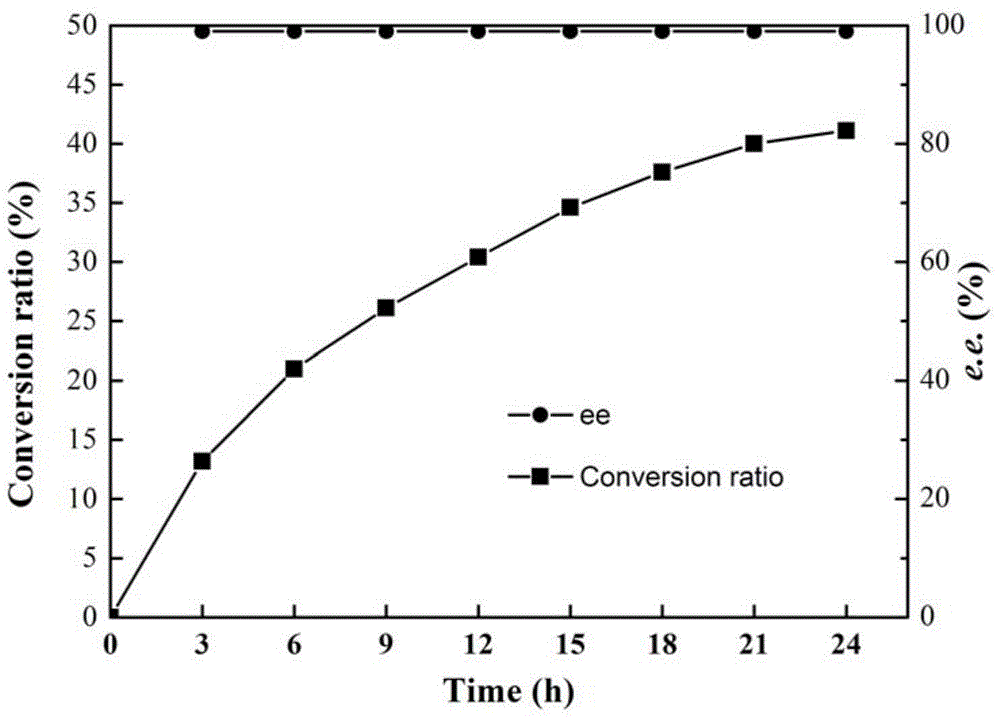
[0031] Pick the single clones in the mutant library in Example 1 and place them on a 96-well plate, add 200 μl of LB medium (containing 50 μg / ml kanamycin) to each well, and culture at 37°C for about 3 hours until the OD 600 About 0.6, add 0.1mM IPTG (isopropyl-β-D-thiogalactopyranoside), and induce at 28°C for 10h. 4000g, 4°C, centrifuge for 10min, discard the supernatant, resuspend the cells with PBS buffer (pH7.2, 10mM), add the substrate containing 2-carboxyethyl-3-cyano-5-methylhexanoic acid ethyl The reaction solution of the ester and the indicator bromothymol blue was used as a reference to the engineered bacteria cells before mutation, and the positive clones with improved activity were initially screened on a 96-well plate. The positi...
Embodiment 3
[0032] Example 3: Site-directed mutagenesis of S63L and D232A
[0033] Refer to the description of site-directed mutagenesis technology (Current Protocols in Protein Science 2011, 26.6.1-26.6.10; Anal. Biochem. 2008, 375: 376-378), the specific process is as follows:
[0034] In order to site-directly mutate Lip-T into single-point mutants S63L and D232A, the mutation primers S63L-F and S63L-R, D232A-F and D232A-R were designed respectively (Table 1), and the plasmid pET28-Lip-T was used as a template , for whole plasmid amplification for site-directed mutagenesis. The PCR system is: 5×PS Buffer 10 μl, dNTP (2.5mM each) 4 μl, mutation primers S63L-F and S63L-R (or D232A-F and D232A-R) 0.5 μl each, plasmid pET28-Lip-T 0.5 μl, PrimeSTAR DNA polymerase 0.5 μl, rehydrate to 50 μl. PCR conditions were pre-denaturation at 98°C for 2 minutes, 25 cycles: 98°C for 10s, 65°C for 10s, 72°C for 6min, and finally 72°C for 10min. After the PCR was positive by 0.9% agarose gel electrophor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com