A kind of sn base hydroxide type photocatalyst and its preparation method and application
A photocatalyst and hydroxide technology, applied in metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, chemical instruments and methods, etc. Complex preparation process, low benzene activity and other problems, to achieve the effect of high activity stability, simple preparation process, and improved degradation rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] This embodiment prepares MgSn(OH) according to the following steps 6 and ZnSn(OH) 6 :
[0030] a. Weigh 0.2mol (56.948g) Na with an electronic balance 2 SnO 3 4H 2 O, add deionized water to dissolve at 10°C, and then set the volume in a 2000mL volumetric flask to obtain Na with a concentration of 0.1mol / L 2 SnO 3 solution;
[0031]Weigh 0.05mol (10.165g) MgCl with an electronic balance 2 ·6H 2 O, add deionized water to dissolve at 10°C, and then set the volume in a 500mL volumetric flask to obtain MgCl with a concentration of 0.1mol / L 2 solution;
[0032] Weigh 0.05mol (6.815g) ZnCl with an electronic balance 2 , add deionized water to dissolve at 10°C, and then set the volume in a 500mL volumetric flask to obtain ZnCl with a concentration of 0.1mol / L 2 solution.
[0033] b. Take two groups of 20mL above-mentioned Na 2 SnO 3 The solution was placed in a constant pressure dropping funnel, and was added dropwise to 20mL MgCl at 10°C under vigorous stirring a...
Embodiment 2
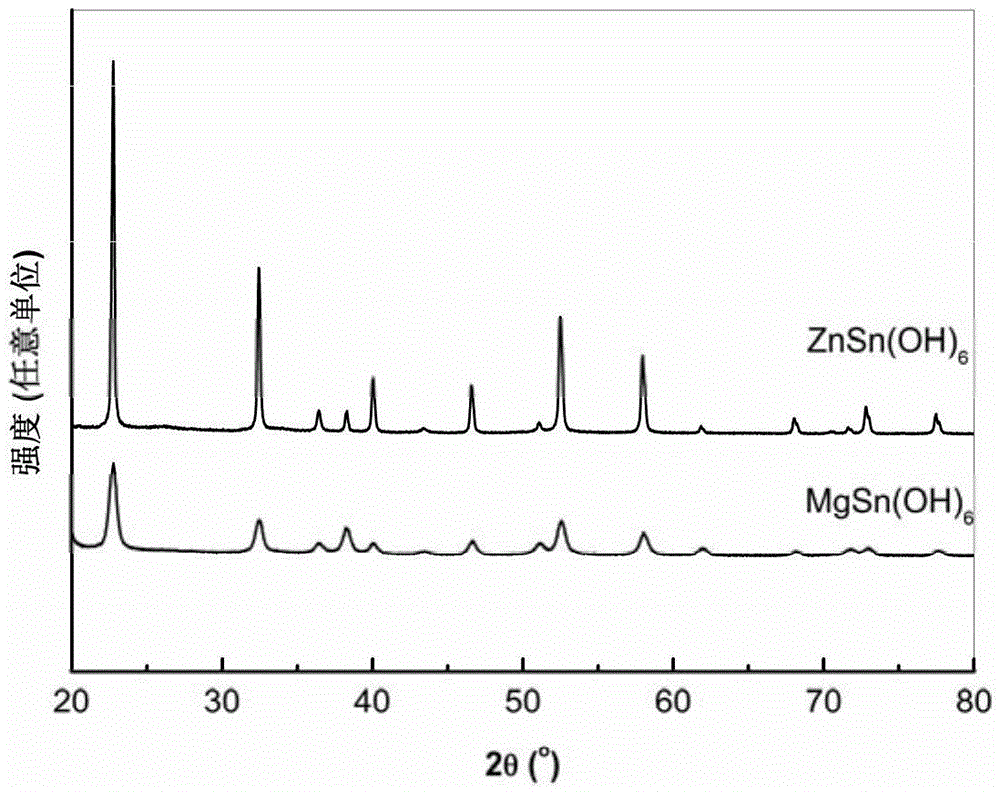
[0041] In order to compare the influence of the hydrothermal reaction temperature on the performance of the photocatalyst, the present embodiment prepared MgSn(OH) by the same steps as in Example 1 6 and ZnSn(OH) 6 , the only difference is that the hydrothermal reaction temperature was changed to: 20°C, 90°C and 150°C respectively. From Figure 4 It can be seen that the XRD of the obtained product is not significantly different from that obtained at 120°C, both of which are cubic phases.
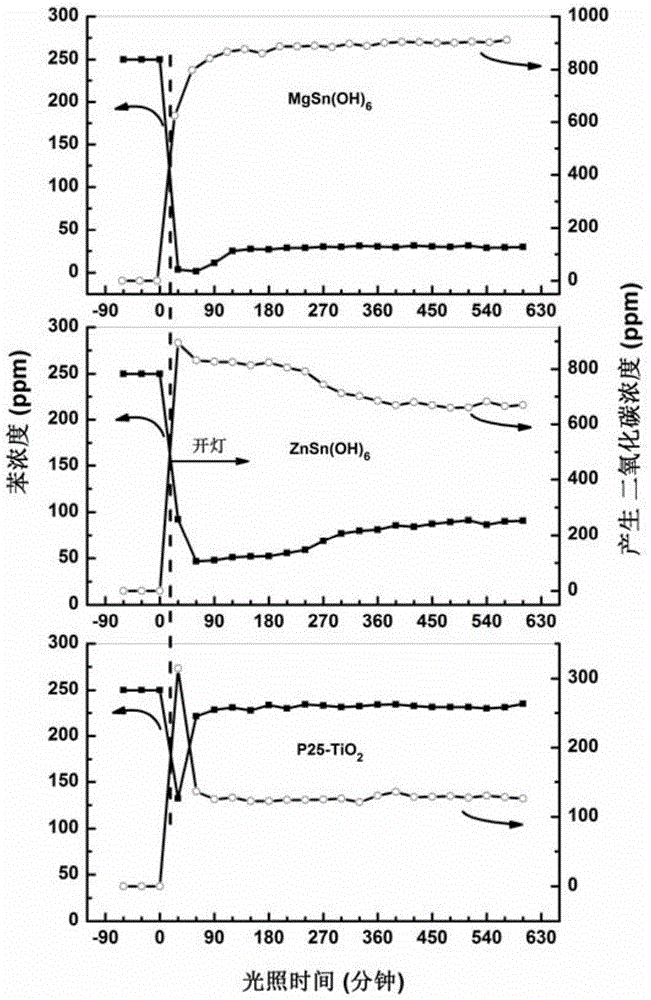
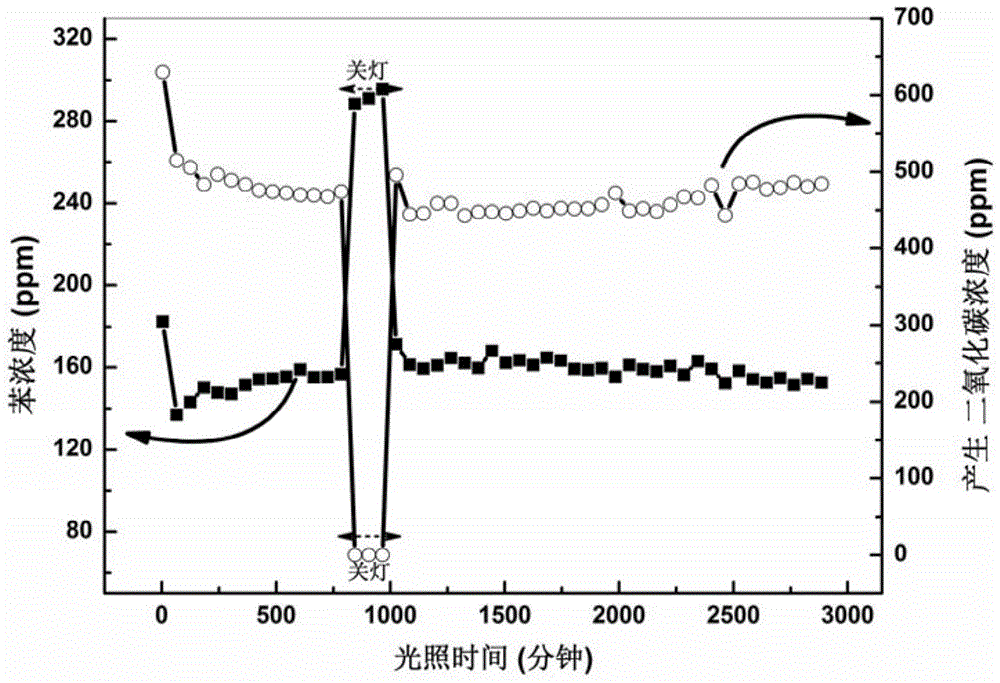
[0042] With ZnSn(OH) 6 For example, its degradability is tested in the same manner as in Example 1, and the result statistics are as follows: Figure 5 As shown, it can be seen from the figure that the degradation rate of the product obtained at any temperature is much higher than that of the P25 catalyst, and the product obtained at 120 ° C is the best, and each product also has a relatively high temperature. Good mineralization rate.
[0043] The obtained MgSn(OH) at each temperature ...
Embodiment 3
[0045] In order to compare the influence of the pH value of the solution on the performance of the photocatalyst, the present embodiment prepared MgSn(OH) by the same steps as in Example 1 6 and ZnSn(OH) 6 , the difference is only in the Na 2 SnO 3 The solution was added dropwise to MgCl 2 solution and ZnCl 2 After the solution, before the hydrothermal reaction, the pH of the mixed solution was adjusted to 4 and 7 with 0.1 mol / L hydrochloric acid, and the pH of the mixed solution was adjusted to 13 with 0.1 mol / L NaOH solution. From Figure 6 It can be seen that the resulting ZnSn(OH) 6 The XRD of the product obtained under the pH condition of Example 1 is basically the same as that of the product obtained in the cubic phase. And the obtained MgSn(OH) 6 When the product is at pH 7 or 13, the XRD of the product obtained under the pH condition of Example 1 is basically the same as that of the cubic phase; when the pH is 4, SnO 2 Miscellaneous.
[0046] With ZnSn(OH) 6 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com