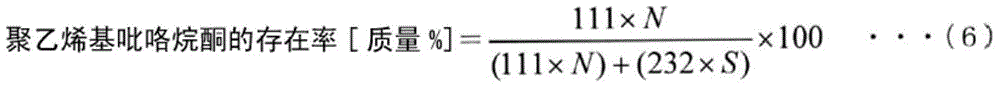Blood processing hollow fiber membrane, blood purifier and manufacturing method thereof
A blood treatment and fibrous membrane technology, applied in chemical instruments and methods, membrane technology, suction devices, etc., can solve the problems of reduced permeability, large changes in performance reduction ratios, and reduced performance, and achieves the effect of less performance changes.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
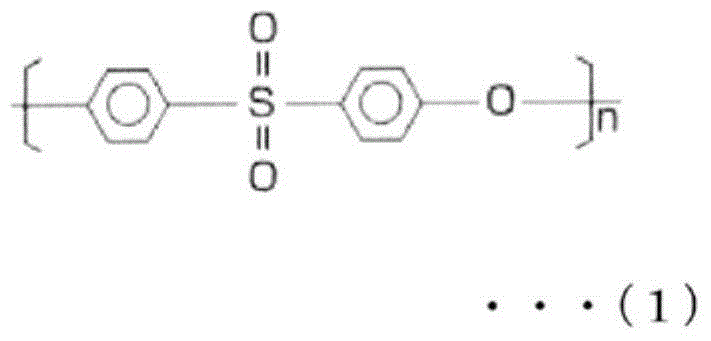
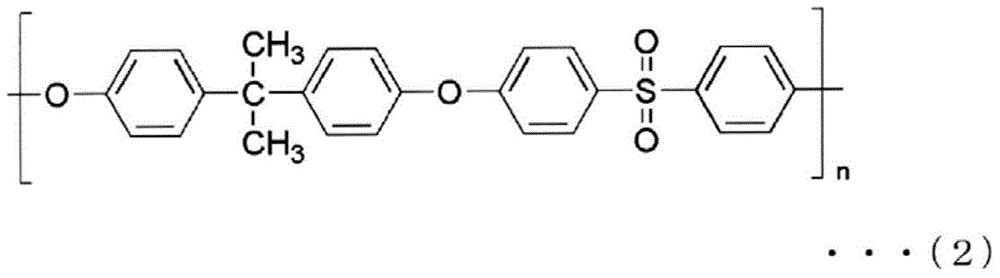
[0284] 17.5% by mass of polyethersulfone (PES) (4800P manufactured by Sumitomo Chemical Co., Ltd.), 3.5% by mass of polyvinylpyrrolidone (PVP) (K90 manufactured by BASF JAPAN LTD.) as a hydrophilic agent, and 1.0% of water as a non-solvent % by mass, triethylene glycol (TEG) (manufactured by Mitsubishi Chemical Co., Ltd.) 31.2 mass%, dimethylacetamide (DMAc) as a solvent (manufactured by Mitsui Chemicals Co., Ltd.) 46.8 mass % of the spinning dope was maintained at 45 The outer slit of the double-layer spinneret of ℃ is ejected, and the water as the internal liquid is ejected from the inner ejection hole of the double-layer spinneret, and it passes through the air gap with an air gap length of 600mm and a spinning speed of 60m / min. , immersed in a 70°C coagulation bath (DMAc:TEG:water=6:4:90).
[0285] Thereafter, it was washed in pure water at 45° C. for 1 minute and in pure water at 80° C. for 90 seconds, and wound up on a straw to obtain a hollow fiber membrane with an inne...
Embodiment 2
[0293] In the spinning dope, PVP (K90 manufactured by BASF JAPAN LTD.) was 3.0% by mass, the solvent dimethylacetamide (DMAc) (manufactured by Mitsui Chemicals Co., Ltd.) was 47.3% by mass, and the vitamin E concentration of the covering solution was 0.45% by mass , except that, a blood purifier was obtained in the same manner as in Example 1.
[0294] The content of vitamin E on the surface of the hollow fiber membrane in the obtained blood purifier is 150mg / m 2 , the ratio of vitamin E present on the inner surface to the vitamin E present in the entire hollow fiber membrane is 70% by mass, and the ratio of polyvinylpyrrolidone (PVP) present on the inner surface of the hollow fiber membrane to PES and polyvinylpyrrolidone ( The ratio of the sum of PVP) was 25% by mass, and the amount of IPA contained in 1 g of the hollow fiber membrane was 950 μg.
Embodiment 3
[0296] In the spinning stock solution, polyvinylpyrrolidone (PVP) (K90 manufactured by BASF JAPAN LTD.) was 3.3% by mass, and the solvent dimethylacetamide (DMAc) (manufactured by Mitsui Chemicals, Ltd.) was 47.0% by mass.
[0297] As the coating solution, a 57% by mass aqueous solution of isopropyl alcohol in which 0.45% by mass of vitamin E was dissolved was used. After the coating solution was passed through the inner cavity of the hollow fiber membrane at a temperature of 20°C, the air was flashed immediately to remove the residual liquid in the inner cavity, and then ventilated with dry air at 24°C in an isopropanol atmosphere for 45 minutes to dry out the solvent. The other conditions were the same as in Example 1 to obtain a blood purifier.
[0298] The content of vitamin E on the surface of the hollow fiber membrane in the obtained blood purifier is 150mg / m 2 , the ratio of the vitamin E present on the inner surface to the vitamin E present in the entire hollow fiber...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The inside diameter of | aaaaa | aaaaa |
| Film thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com