A class of nitrogen azole compounds and its preparation method and application
A technology for compound and azole, which is applied in the field of medicine and achieves the effects of high yield, simple preparation method and good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The preparation of embodiment 1 compound of the present invention
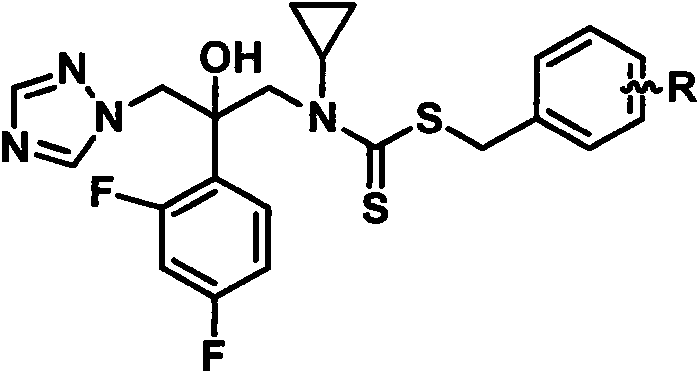
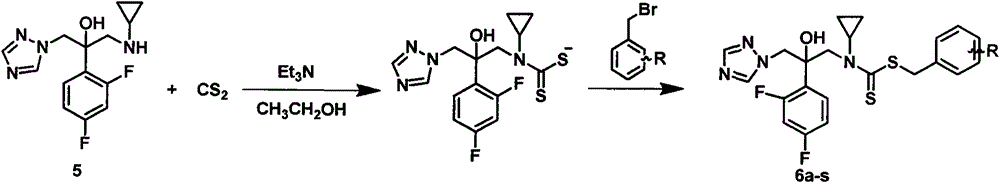
[0031] (1) 1-(1H-1,2,4-triazol-1-yl)-2-(2,4-difluorophenyl)-3-(N-cyclopropylamino)-2-ol
[0032] 1-[2-(2,4-difluorophenyl)-2,3-epoxypropyl]-1H-1,2,4-triazole mesylate 21g, with cyclopropylamine 10mL, triethylamine 20mL, heat and reflux in 300mL ethanol for 6-8 hours, distill off the solvent after the reaction is complete, extract with 200mL ethyl acetate, wash with 100mL×2 water, dry over anhydrous sodium sulfate, filter, distill off ethyl acetate to obtain oily 1- (1H-1,2,4-triazol-1-yl)-2-(2,4-difluorophenyl)-3-(N-cyclopropylamino)-2-ol 11.96g, yield 68.0 %.
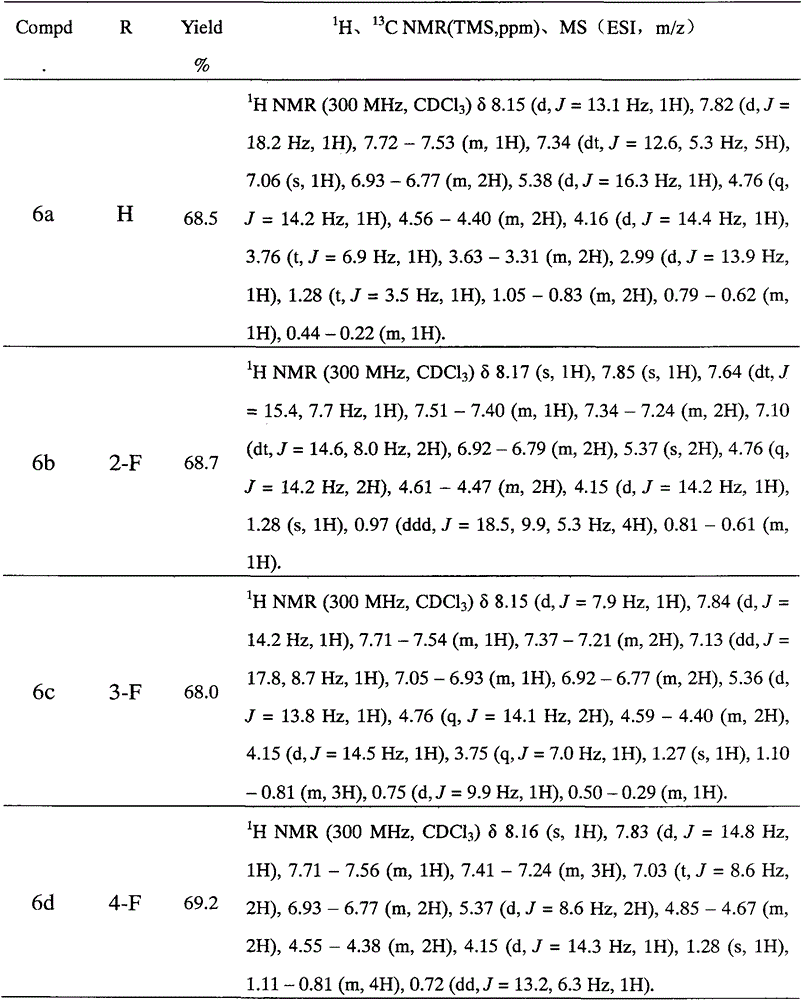
[0033] (2) Preparation of N-methyl-N-(2-(2,4-difluorophenyl)-2-hydroxyl-3-(1H-1,2,4-triazol-1-yl))-propane Benzyl-cyclopropylcarbamate (compound 6a in Table 1):
[0034] In a 50mL single-neck bottle, put a magnet, add 10mL absolute ethanol, 0.536g 1-(1H-1,2,4-triazol-1-yl)-2-(2,4-difluorophenyl)- 3-(N-Cyclopropylamino)-2-ol (2 mmol). After...
Embodiment 2
[0079] The pharmacological experiment of embodiment 2 compounds of the present invention
[0080] (1) Experimental method
[0081] Conventional in vitro antibacterial test methods are used, see: Antimicrob Agents Chemother 1995, 39 (5): 1169 for details.
[0082] (1) Experimental strains
[0083] In this experiment, the following 8 common human pathogenic standard fungal strains were selected as screening objects:
[0084] Deep fungi: Candida albicans, Cryptococcus neoformans, Candida glabrata, Candida parapsilosis;
[0085] Superficial fungi: Trichophyton rubrum;
[0086] Subcutaneous fungi: Microsporum gypsum, Aspergillus fumigatus.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com