Azole antifungal compound and its preparation method and application
A compound and antifungal technology, applied in antifungal agents, organic chemistry, pharmaceutical formulations, etc., can solve unseen problems, achieve good effect, high yield, and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
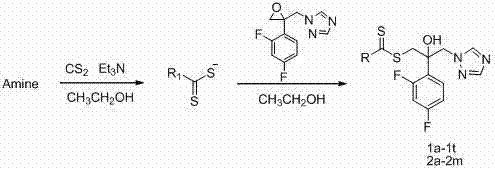
[0030] Embodiment 1 Preparation of the compound of the present invention
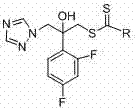
[0031] (1) Preparation of 2-(2,4-difluorophenyl)-2-hydroxyl-3-(1 H -1,2,4-triazol-1-yl)diethylaminodithiocarbamate (compound 1k in Table 1):
[0032] 1-[2-(2,4-difluorophenyl)-2,3-epoxypropyl]-1 H 10g of -1,2,4-triazole mesylate was dissolved in 100mL ethyl acetate and 100mL saturated sodium carbonate solution, then shaken and separated, and the organic layer was washed once with 50mL saturated sodium carbonate solution and 50mL saturated sodium chloride solution. , dried over anhydrous sodium sulfate. Filtration, evaporation of the solvent to give intermediate 1-[2-(2,4-difluorophenyl)-2,3-epoxypropyl]-1 H -1,2,4-triazole.
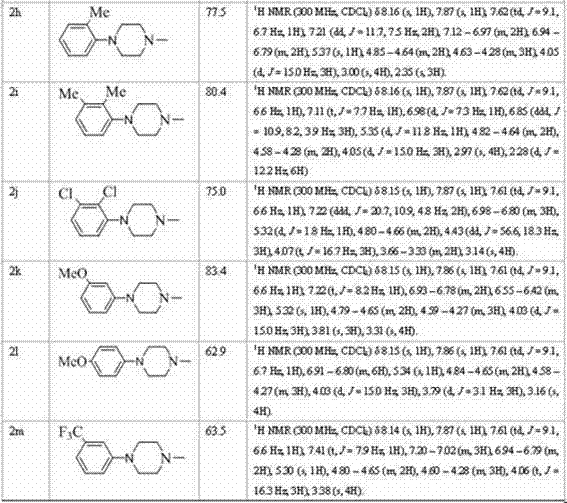
[0033] Put a magnet into a 50mL single-necked bottle, add 10mL absolute ethanol and 0.21mL diethylamine (2mmol). After 10 min of ice-water bath, 0.12 mL of carbon disulfide (2 mmol) was added dropwise under rapid magnetic stirring, and kept stirring in the ice bath for 30 min...
Embodiment 2
[0076] Embodiment 2 Pharmacological experiments of compounds of the present invention
[0077] (1) Experimental method
[0078] Conventional in vitro antibacterial test methods are used, see: Antimicrob Agents Chemother 1995,39(5):1169 for details.
[0079] (1) Experimental strains
[0080] In this experiment, the following 8 common human pathogenic standard fungal strains were selected as screening objects:
[0081] Deep fungi: Candida albicans, Cryptococcus neoformans, Candida glabrata, Candida parapsilosis;
[0082] Superficial fungi: Trichophyton rubrum;
[0083] Subcutaneous fungi: Microsporum gypsum, Aspergillus fumigatus.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com