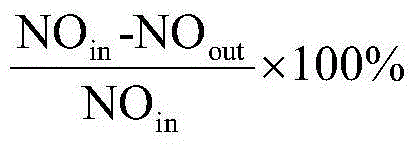Denitrification copper radical molecular sieve catalyst as well as preparation method and application thereof
A molecular sieve and catalyst technology, applied in the field of nitrogen oxide reduction catalyst preparation in environmental protection, can solve problems such as poor hydrothermal stability, achieve high water stability, and improve the effect of hydrothermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
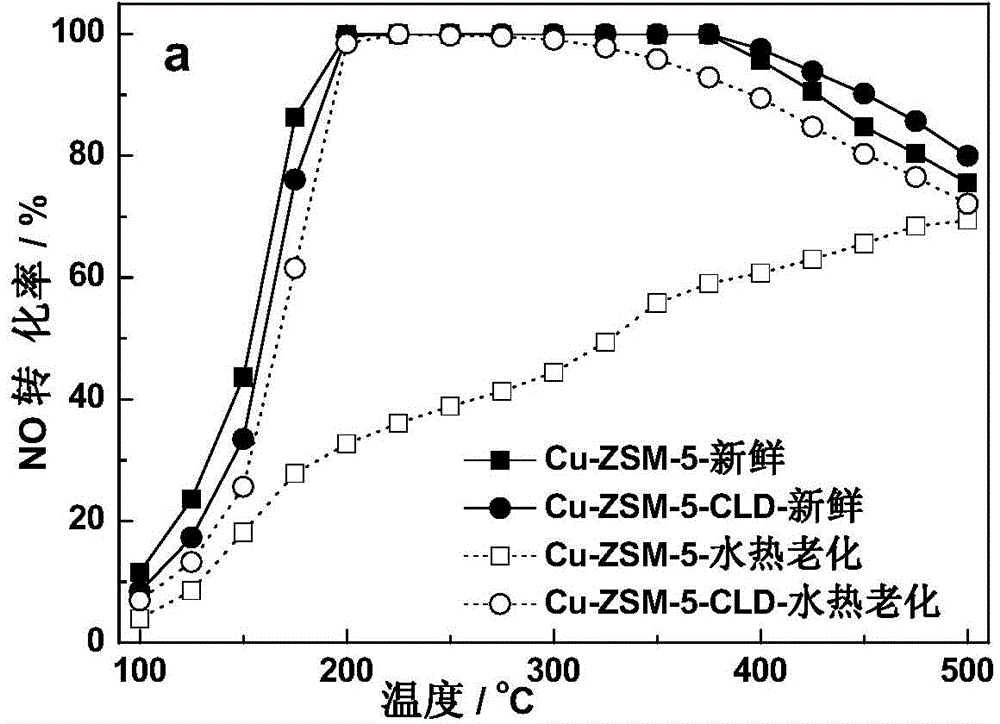
[0056] The preparation of embodiment 1 Cu-ZSM-5-CLD sample
[0057] Add ZSM-5 to ammonium nitrate solution, stir at 80 °C for 1 h, then filter and wash with deionized water to obtain NH 4+ form, and then the resulting NH at 100 °C 4+ / ZSM-5 was dried for 16 hours, and finally the above steps were repeated twice to make the ammonia exchange fully. NH 4+ / ZSM-5 added to Cu(CH 3 COO) 2 The solution was stirred for 8h, in which Cu(CH 3 COO) 2The concentration of Cu should be small to avoid Cu accumulating on the surface and pores of molecular sieves. The resulting samples were then filtered and rinsed with deionized water, then dried at 100 °C for 16 h, and finally calcined at 550 °C for 5 h in an air atmosphere.
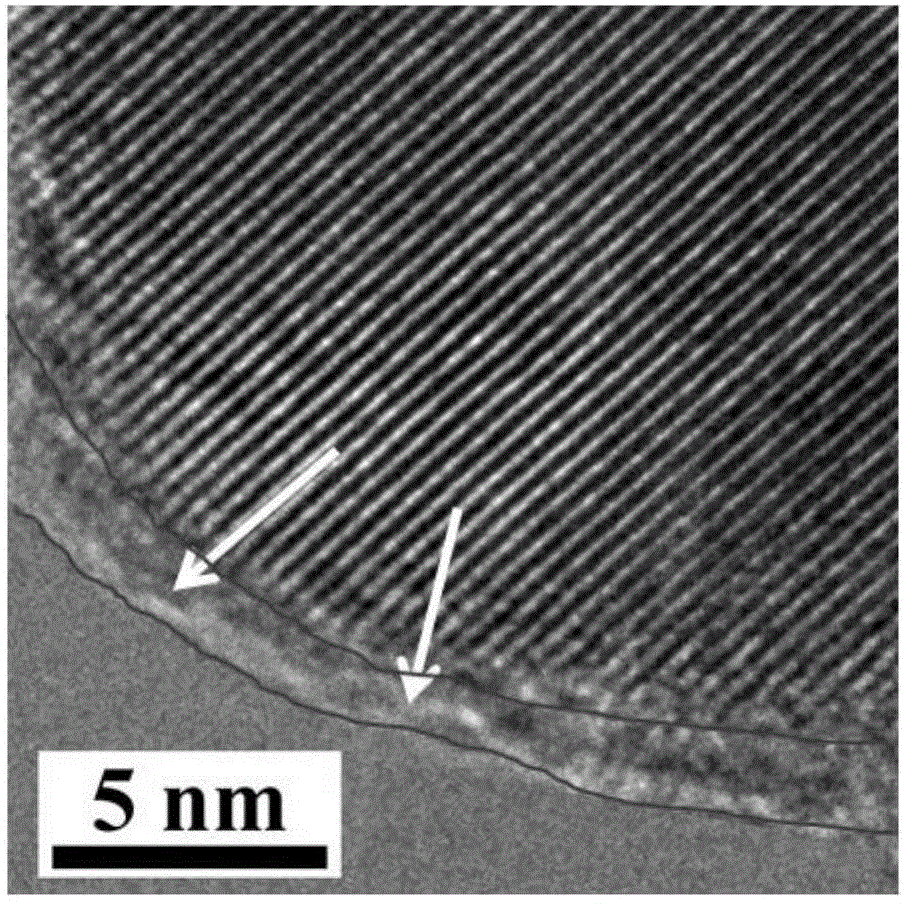
[0058] The surface modification of the Cu-ZSM-5 molecular sieve catalyst prepared above was carried out by using the liquid phase deposition method. The specific process was as follows: 1g of the catalyst was added to 25mL of n-hexane, and then 0.15mL of tetraeth...
Embodiment 2
[0061] The preparation of embodiment 2Cu-MOR sample
[0062] MOR was added to the ammonium nitrate solution, stirred at 80 °C for 1 h, then filtered and washed with deionized water to obtain NH 4+ form, and then the resulting NH at 100 °C 4+ / MOR drying for 16 hours, and finally repeat the above steps twice to make ammonia exchange sufficient. NH 4+ / MOR added to Cu(NO 3 ) 2 The solution was stirred for 8h, in which Cu(NO 3 ) 2 The concentration of Cu should be small to avoid Cu accumulating on the surface and pores of molecular sieves. The resulting samples were then filtered and rinsed with deionized water, then dried at 100 °C for 16 h, and finally calcined at 550 °C for 5 h in an air atmosphere.
[0063] The Cu-MOR molecular sieve catalyst prepared above was surface-modified by the liquid phase deposition method, and the specific process was as follows: 1 g of the catalyst was added to 30 mL of isohexane, and then 0.15 mL of tetraethylorthosilicate was added thereto...
Embodiment 3
[0064] The preparation of embodiment 3Cu-BEA sample
[0065] BEA was added to the ammonium nitrate solution, stirred at 80 °C for 1 h, then filtered and washed with deionized water to obtain NH 4+ form, and then the resulting NH at 100 °C 4+ / BEA was dried for 16 hours, and finally the above steps were repeated twice to make the ammonia exchange sufficient. NH 4+ / BEA added to CuSO 4 The solution was stirred for 8h, in which CuSO 4 The concentration of Cu should be small to avoid Cu accumulating on the surface and pores of molecular sieves. The resulting samples were then filtered and rinsed with deionized water, then dried at 100 °C for 16 h, and finally calcined at 500 °C for 4 h in an air atmosphere.
[0066] The surface of Cu-BEA molecular sieve catalyst prepared above was modified by liquid phase deposition method. The specific process was as follows: 1 g of catalyst was added to 25 mL of n-pentane, and then 0.20 mL of tetraethylorthosilicate was added thereto. Then...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com