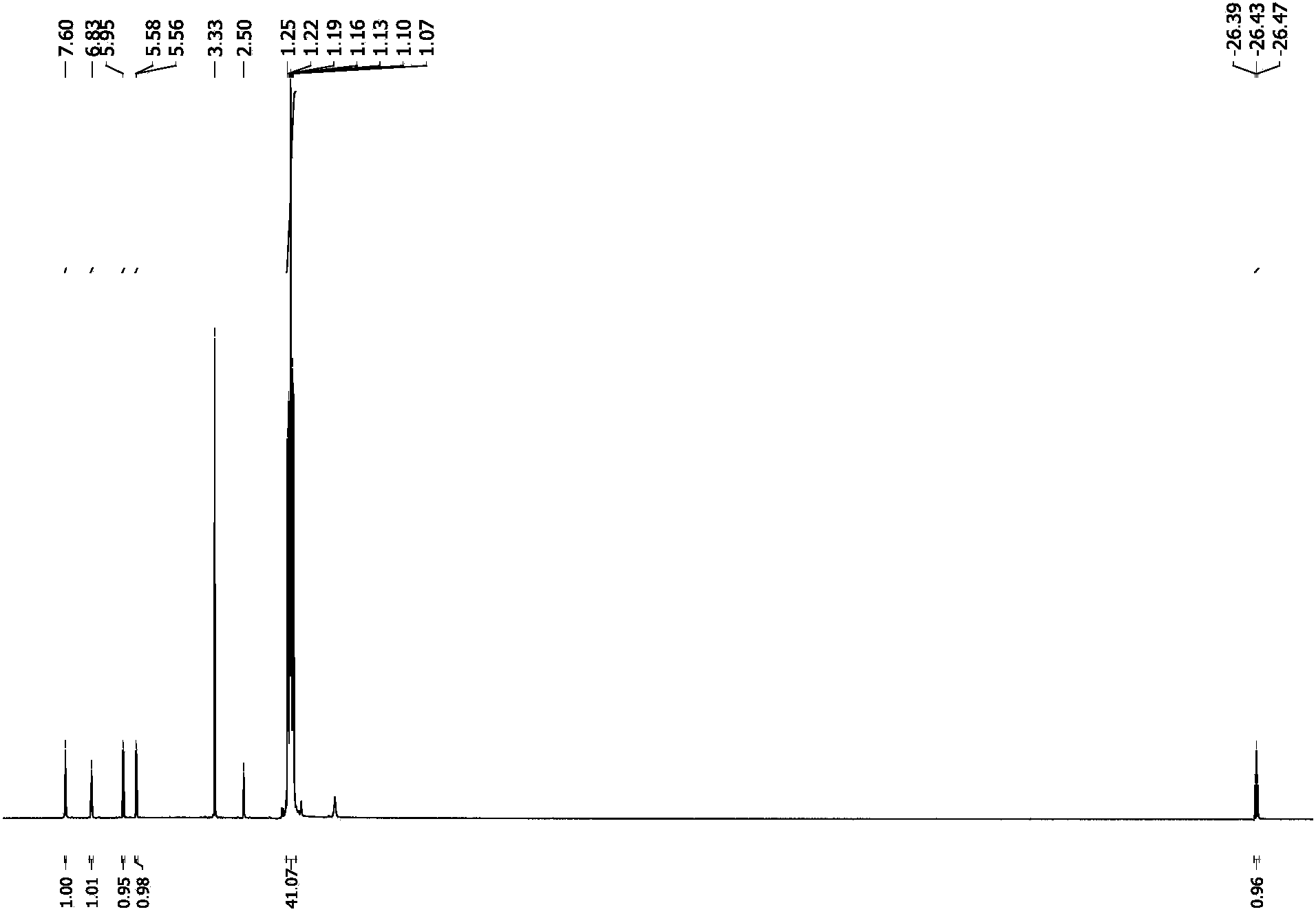Ruthenium catalyst capable of efficiently catalyzing decomposing of formic acid for preparing hydrogen
A technology of catalytic decomposition, ruthenium catalyst, applied in physical/chemical process catalyst, organic compound/hydride/coordination complex catalyst, hydrogen and other directions, can solve the problem of low hydrogen conversion rate or high pressure, high reaction temperature, Toxic problems such as fuel cells, to achieve the effect of reducing conditions, simple preparation process, good activity and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
[0041] Embodiment case 1: the preparation of ruthenium catalyst.
[0042] Step 1) combine Pincer compound with HRuCl(PPh 3 ) 3 Mix in equal proportions and react at 65°C for 12 hours to obtain the catalytic precursor Ru1.
[0043] Step 2) Add alkali KOBu to the Ru1 prepared in step 1) in a moderate proportion t , react at room temperature for 2 hours, and the ruthenium catalyst Ru Cat. can be prepared.
[0044]
Embodiment example 2
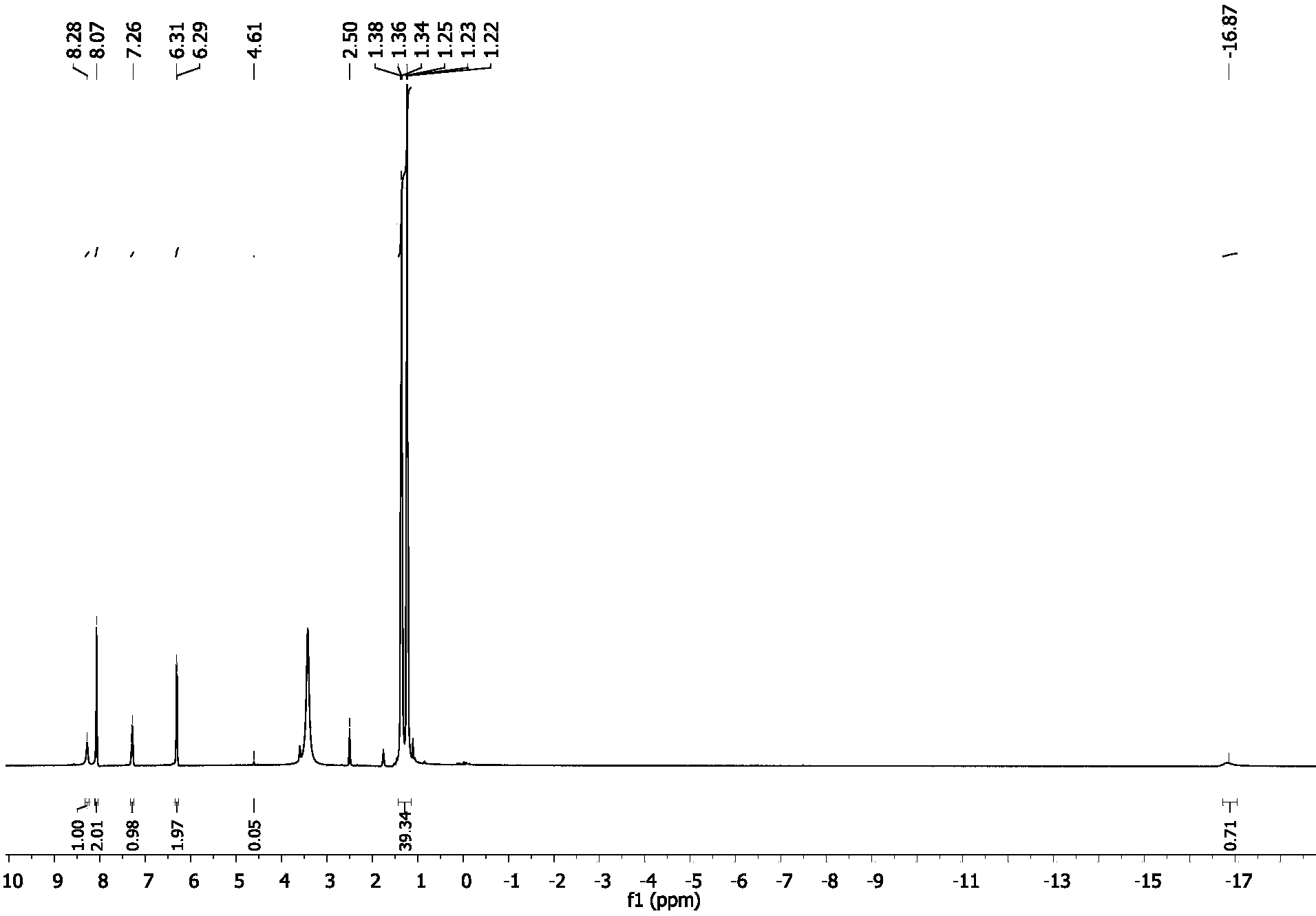
[0045] Implementation Case 2: The process of catalyzing the decomposition of formic acid to prepare hydrogen.
[0046] Step 1) will 10 -3 The mmol ruthenium catalyst was dissolved in 5ml DMSO solvent, added to the reaction system equipped with formic acid solution, and reacted continuously under the temperature conditions of 20, 50, and 80°C respectively to obtain gas continuously.
[0047] Step 2) pass the prepared mixed gas into the KOH solution to remove CO 2 , the resulting gas is hydrogen.
Embodiment example 3
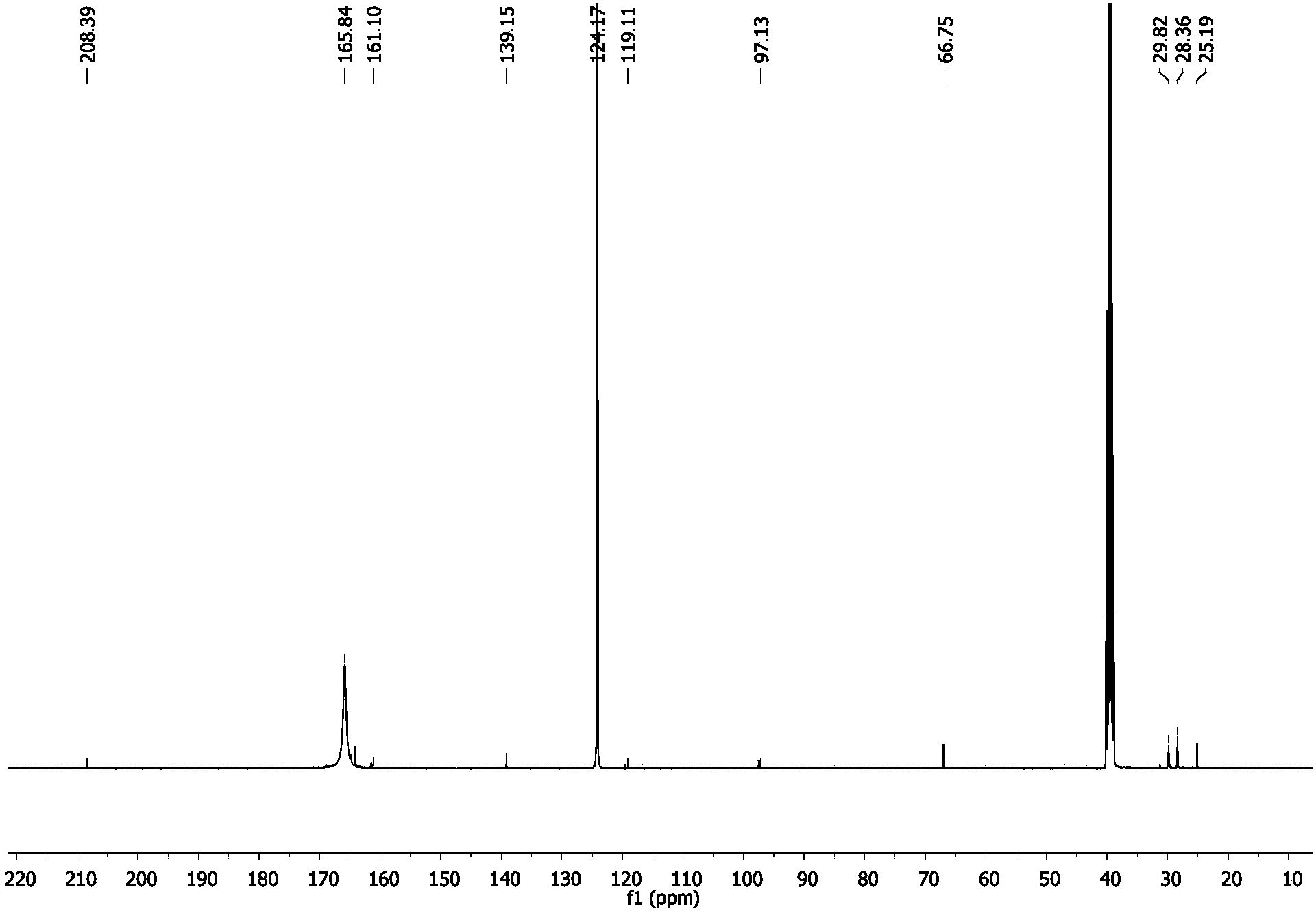
[0048] Implementation case 3: the influence of catalyst solvent on the generation of hydrogen from formic acid.
[0049] The catalyst obtained in embodiment case 1 can preferably CH 3 CN, DMF, thf, thf / H 2One of O and DMSO is used as a solvent. For this reason, various solvents are used to dissolve the catalyst and the experiment is carried out according to the implementation case 2 under the condition of 50 ° C. The TON value of the catalyst obtained through the experimental results is used as a reference standard to select the preferred solvent. The specific data is shown in the following table :
[0050] Table 1 is the TON of the catalyst under the conditions of 50 ℃ various solvents
[0051]
[0052] By comparing the data in the table, the TON value of the catalyst can reach 95,000 when DMSO is used as the solvent, so DMSO is preferred as the catalyst solvent.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com