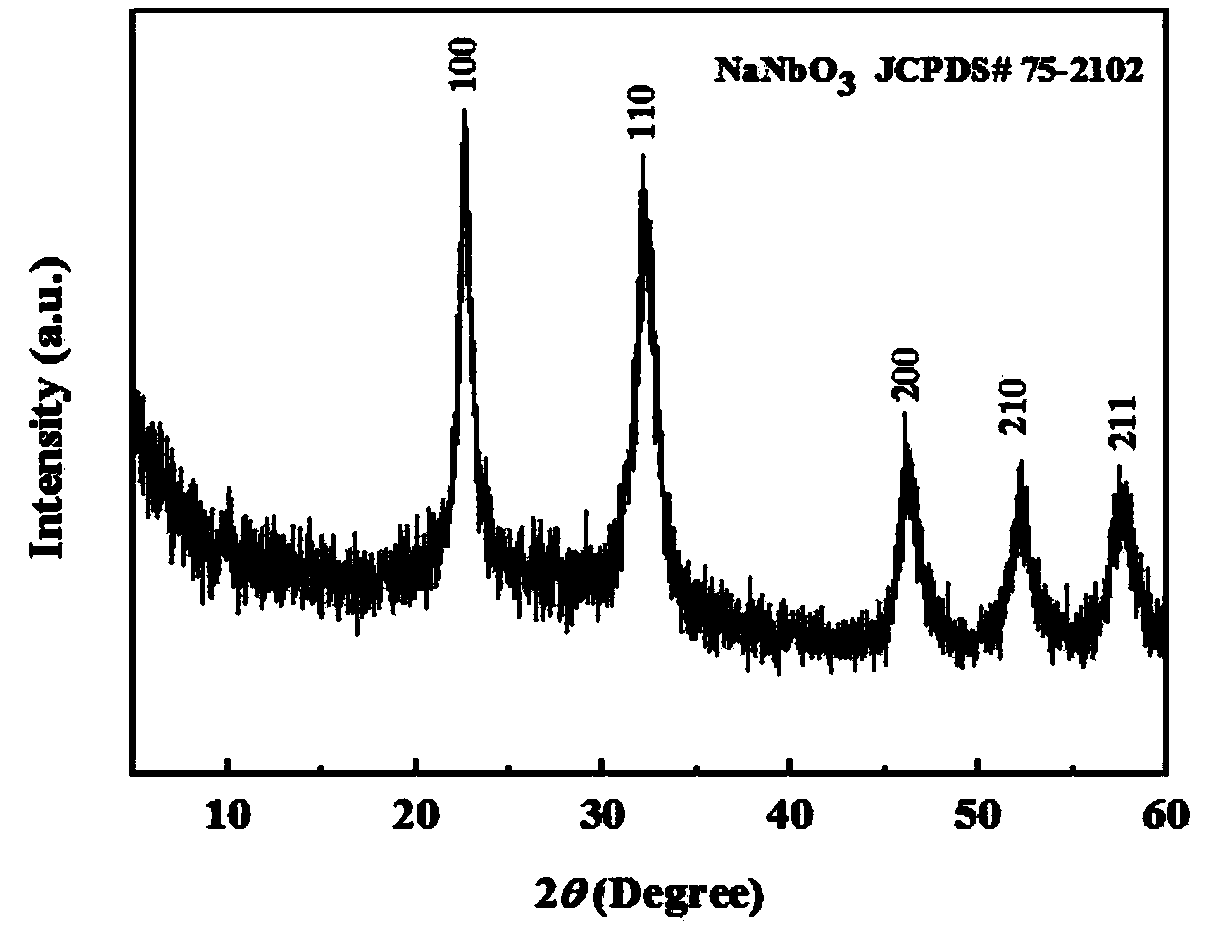
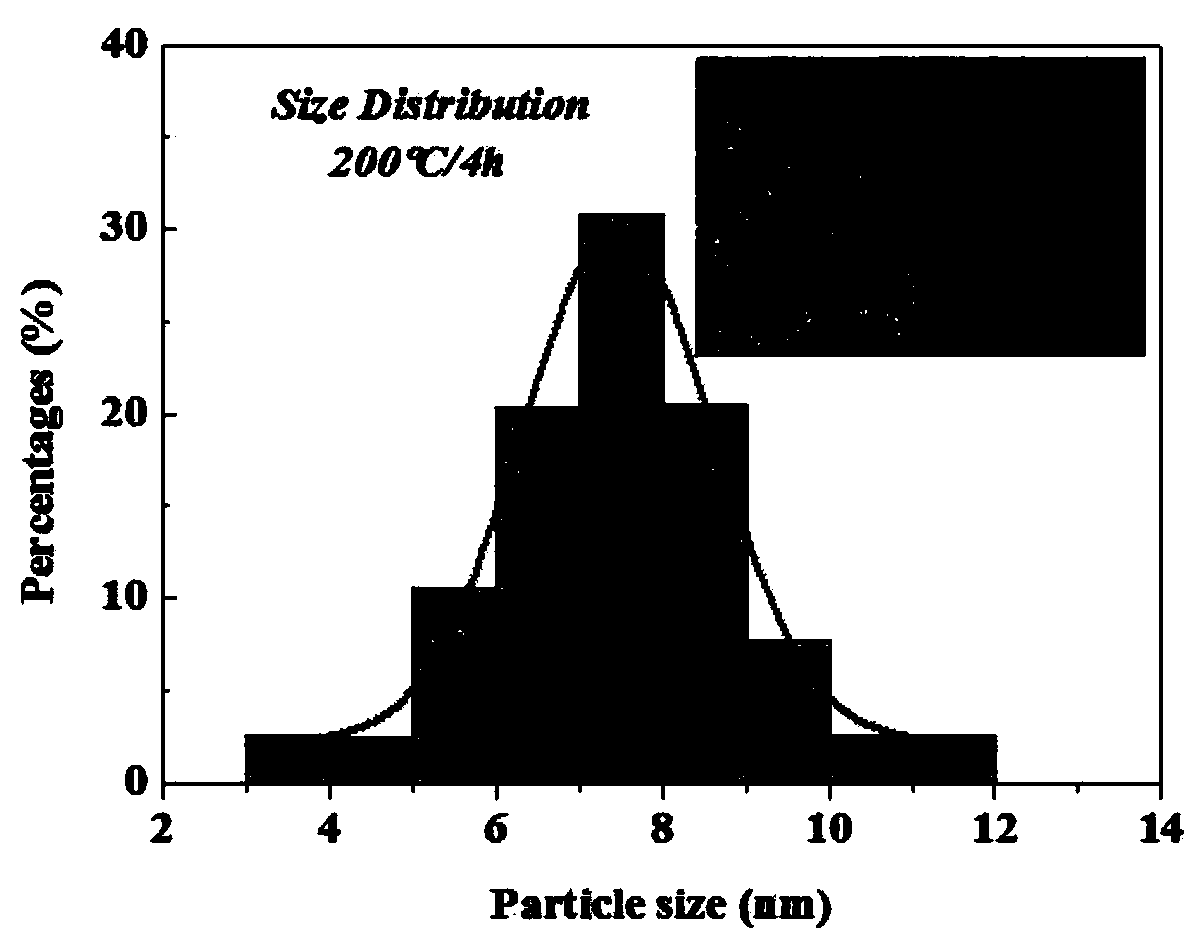
Synthetic method of sodium niobate nanowire
A synthesis method and technology of sodium niobate, applied in niobium compounds, nanotechnology, nanotechnology and other directions, can solve the problems of high energy consumption, low production efficiency, unfavorable industrialization, etc., achieve low energy consumption, improve production efficiency, and be easy to operate. and control effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Add 1.6664g NaOH to 50ml ethylene glycol solvent, heat and stir for 20~30min; after it is fully mixed, continue to add 1.5g raw material Nb 2 O 5 , heating and stirring for 15~20min to make it evenly mixed to form a milky white mixed solution. The obtained mixed solution was transferred to a polytetrafluoroethylene lining, and then the lining was placed in a stainless steel hydrothermal reaction kettle to be sealed, and the heat preservation reaction was carried out at 140° C. for 8 h, and after the reaction was completed, it was naturally cooled to room temperature. The obtained white precipitated product was washed and centrifuged multiple times with deionized water and anhydrous ethanol. The rotating speed was 3000 rpm and the time was 10 min to ensure that all residual ions and organic solvents were washed away; drying treatment was carried out at 60 °C for 24 h. , to obtain sodium niobate (NaNbO 3 ) nanowire powder.
Embodiment 2
[0031] Add 1.6664g NaOH to 50ml ethylene glycol solvent, heat and stir for 20~30min; after it is fully mixed, continue to add 1.0g raw material Nb 2 O 5, heating and stirring for 15~20min to make it evenly mixed to form a milky white mixed solution. The obtained mixed solution was transferred to a polytetrafluoroethylene liner, and then the liner was placed in a stainless steel hydrothermal reactor to seal, and the reaction was kept at 180° C. for 8 hours, and cooled to room temperature naturally after the reaction was completed. The obtained white precipitated product was washed and centrifuged multiple times with deionized water and absolute ethanol, and the rotation speed was 3000 rpm and the time was 10 min to ensure that all residual ions and organic solvents were washed away; 24h drying treatment to obtain sodium niobate (NaNbO 3 ) nanowire powder.
Embodiment 3
[0033] Add 3.3328g NaOH to 50ml ethylene glycol solvent, heat and stir for 20~30min; after it is fully mixed, continue to add 0.5g raw material Nb 2 O 5 , heating and stirring for 15~20min to make it evenly mixed to form a milky white mixed solution. The obtained mixed solution was transferred to a polytetrafluoroethylene lining, and then the lining was placed in a stainless steel hydrothermal reactor to seal, and the reaction was kept at 200° C. for 4 h, and cooled to room temperature after the reaction was completed. The obtained white precipitated product was washed and centrifuged multiple times with deionized water and absolute ethanol, and the rotation speed was 3000 rpm and the time was 10 min to ensure that all residual ions and organic solvents were washed away; After 24h drying treatment, sodium niobate (NaNbO3) nanowire powder was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com