Optical material polymer composition containing thioepoxy compound and optical material manufacturing method
A technology of thioepoxy and optical materials, which is applied in optics, optical components, instruments, etc., can solve the problems of difficult hardening, low lens quality, unhardened polymerization of optical materials, etc., and achieve the goal of increasing yield and reducing production costs Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
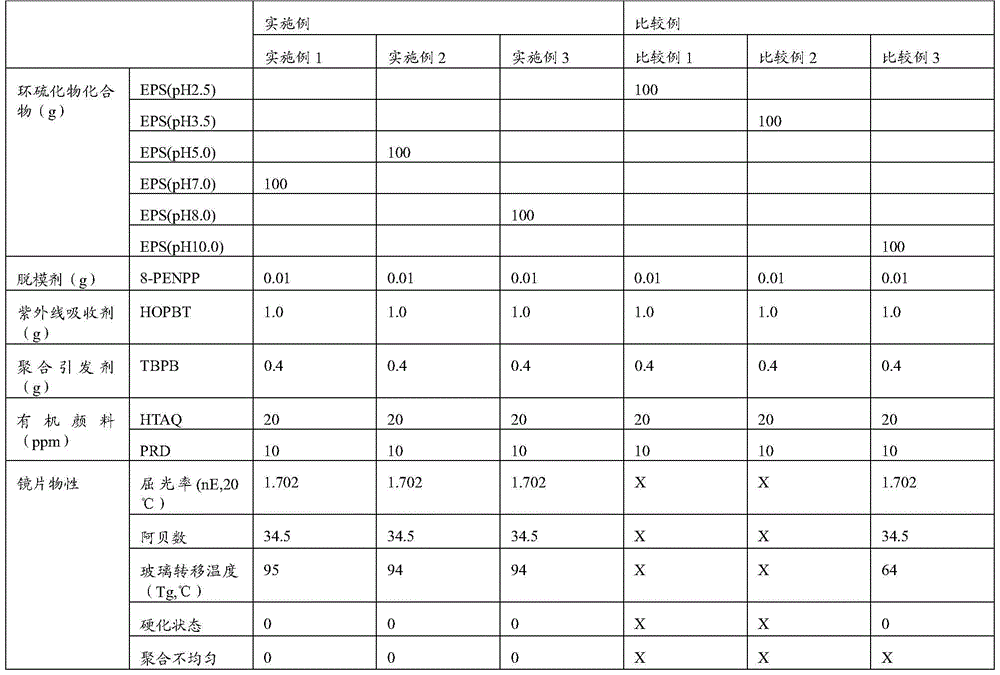
Examples
Synthetic example 1
[0041] Synthesis Example 1: Bis(3-chloro-2-hydroxylpropyl) sulfide (bis(3-chloro-2-hydroxylpropyl) sulfide) synthesis
[0042] After adding epichlorohydrin (5563g, 60.12mol) and methanol (2500g) to a 10-liter reactor, the reaction temperature was adjusted to 6°C. When the reaction temperature reached 6°C, caustic soda (50%) was added. aq,5g). Add NaSH.xH to another 10 liter reactor 2 O (70% NaSH, 3660g, 45.75mol), methanol (1000g) and water (500g) are completely melted by stirring, then slowly add hydrochloric acid dropwise and add the generated hydrogen sulfide to the epichlorohydrin solution to obtain di( 3-chloro-2-hydroxypropyl) sulfide. The end point of the reaction is when the final product is confirmed by GC, epichlorohydrin and 3-chloro-2-hydroxy-propyl-1-thiol compound disappear completely, and bis(3-chloro-2-hydroxypropyl) sulfide is formed Point in time. If 3-chloro-2-hydroxy-propyl-1-thiol is present, the relative content is calculated by GC, and the reaction is...
Synthetic example 2
[0043] Synthesis Example 2: Synthesis of bis-(2,3-epithiopropyl) sulfide (pH 2.5)
[0044] Add bis(3-chloro-2-hydroxypropyl) sulfide (1072.48 g, 4.89 mol), toluene (toluene) 1300 g, and methanol 800 g to a 10 liter reaction vessel, stir, and adjust the reaction temperature to 30°C. When reaching 25°C, NaOH (50% aq., 783.08g, 9.78mol) was added dropwise, and the reaction temperature during the dropwise addition was carried out at 35-37°C, and the temperature was maintained and the reaction proceeded. The dripping is carried out within 1 hour, and then the fermentation is kept at 37°C for about 30 minutes. After the fermentation, 2000g of toluene is added and stirred for about 10 minutes for stratification. The supernatant organic matter is washed twice with water. Limit the removal of water, add 400g of methanol to the organic solution with several layers again, stir, add thiourea (1117.65g, 14.30mol) and anhydrous acetic acid (70g) at the reaction temperature of 8℃, adjust the r...
Synthetic example 3
[0045] Synthesis Example 3: Synthesis of bis-(2,3-epithiopropyl) sulfide (pH 3.5)
[0046] Add bis(3-chloro-2-hydroxypropyl) sulfide (1072.48 g, 4.89 mol), toluene (toluene) 1300 g, and methanol 800 g to a 10 liter reaction vessel, stir, and adjust the reaction temperature to 25°C. When reaching 25°C, NaOH (50% aq., 783.08g, 9.78mol) was added dropwise, and the reaction temperature during the dropwise addition was carried out at 35-37°C, and the temperature was maintained and the reaction proceeded. The dripping is carried out within 1 hour, and then the fermentation is kept at 37°C for about 30 minutes. After the fermentation, 2000g of toluene is added and stirred for about 10 minutes for stratification. The supernatant organic matter is washed twice with water. Limit the removal of water, add 400g of methanol to the organic solution with several layers again, stir, add thiourea (1117.65g, 14.30mol) and anhydrous acetic acid (70g) at a reaction temperature of 8°C, adjust the re...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thermal resistance | aaaaa | aaaaa |
| Abbe number | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com