Application of a Nickel-Based Skeleton Metal Catalyst in Hydrazine Decomposition to Hydrogen Production
A framework metal and catalyst technology, which is applied in the application field of nickel-based framework metal catalysts in hydrazine decomposition hydrogen production, can solve the problems of slow hydrogen production, low catalytic activity, and influence on separation steps, and achieve less by-products and hydrogen selection The effect of high stability and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Preparation of Nickel-based Skeleton Metal Catalysts
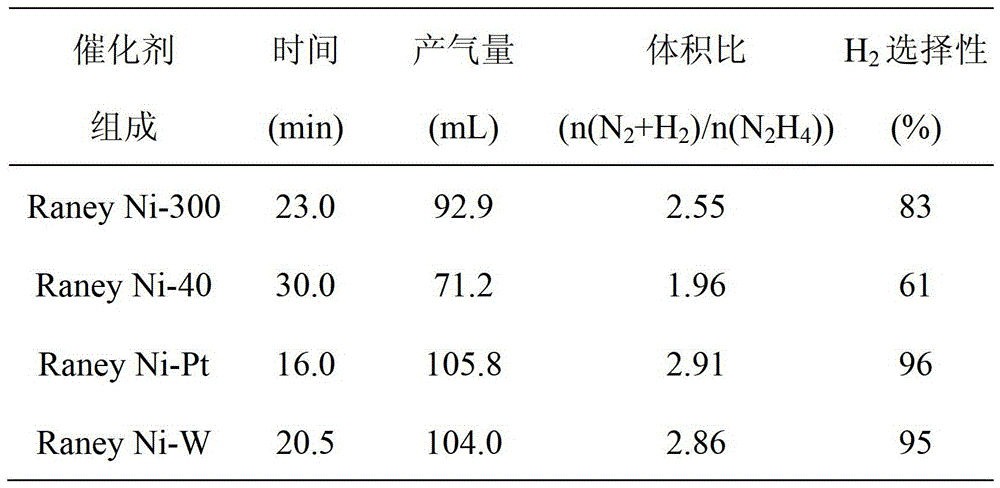
[0025] The catalyst precursor is Ni 50-x al 50-y m x+y , 0≤x≤10, 0≤y≤10 alloys, crushed into 20-300 mesh, added to 5.00mol L -1 In the NaOH solution, stir, the water bath temperature is controlled at 80 ℃, and the reaction time is 3h. After the reaction, the catalyst is repeatedly washed with deionized water until the washing solution is neutral, and then separated by a magnet to obtain a nickel-based skeleton metal catalyst. When x and y are 0 respectively and the alloy powder is 40 mesh, Raney Ni-40 is obtained. When x and y are respectively 0 and the alloy powder is 300 mesh, a Raney Ni-300 catalyst is obtained. When x=2, y=0, and M is Pt, a Raney Ni-Pt catalyst is obtained; when x=1, y=1, and M is W, a Raney Ni-W catalyst is obtained.
Embodiment 2
[0027] Application of Nickel-Based Skeleton Metal Catalysts in Decomposition of Hydrazine
[0028] This reaction was carried out in a closed drainage system, and the experimental process was as follows: first, 4 mL of deionized water and 0.1 g of catalyst were added to the three-necked bottle, and then the system was sealed; then, 0.50 ml of hydrazine hydrate solution was injected into the above-mentioned three-necked bottle, Get an initial concentration of 0.10-0.45mol L -1 The hydrazine solution starts timing simultaneously, and the reaction temperature is between 0-100°C. The gas produced by the catalytic decomposition of hydrazine first passes through the hydrochloric acid absorption device to convert the possible by-product NH 3 Absorption, ensuring that only hydrogen and nitrogen remain. The product gas is collected, the activity of the reaction is calculated by reading the gas production per unit time, and the selectivity of the reaction is calculated by reading the c...
Embodiment 3
[0030] Catalytic Activity Test of Hydrazine Hydrate Decomposition with Different Alkali Concentrations
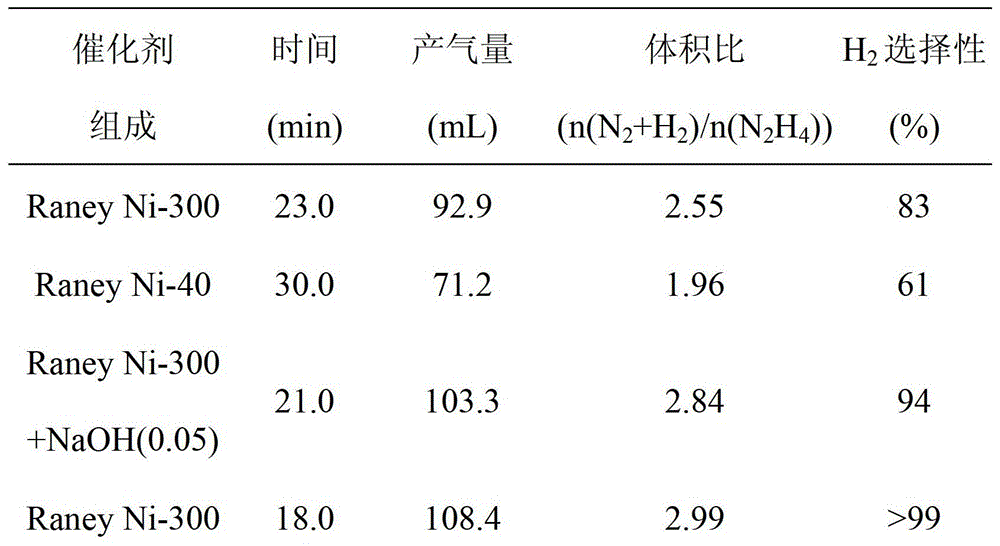
[0031] Using the single active center Raney Ni-300 catalyst prepared in Example 1, add different amounts of NaOH respectively so that its initial concentration in the reaction solution is 0.01-5.00mol L -1 , to obtain Raney Ni-300+NaOH catalytic system, and used in the hydrogen production reaction of hydrazine hydrate decomposition described in Example 2.
[0032]The activity test results of different catalysts are compared, as shown in Table 1.
[0033] Table 1 Test results of hydrazine decomposition hydrogen production of different catalysts (30°C, initial hydrazine concentration is 0.32mol L -1 )
[0034]
[0035]
[0036] It can be seen from Table 1 that the 300-mesh single-active center skeleton nickel catalyst has a selectivity of up to 83% for hydrogen production by decomposition of hydrazine hydrate at room temperature, which is much higher than that of the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com