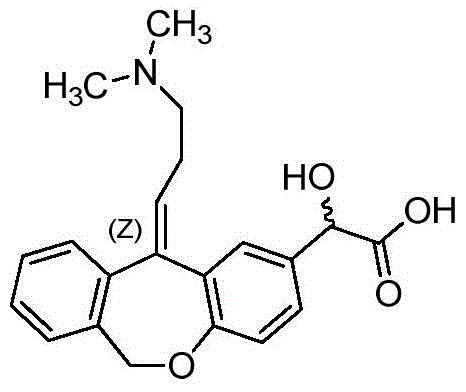
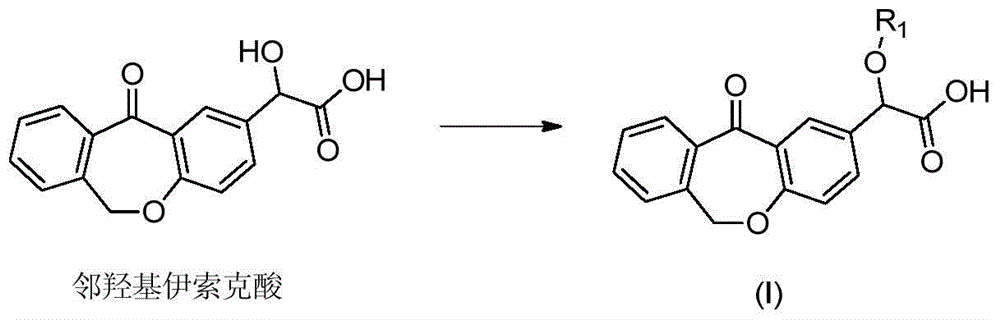
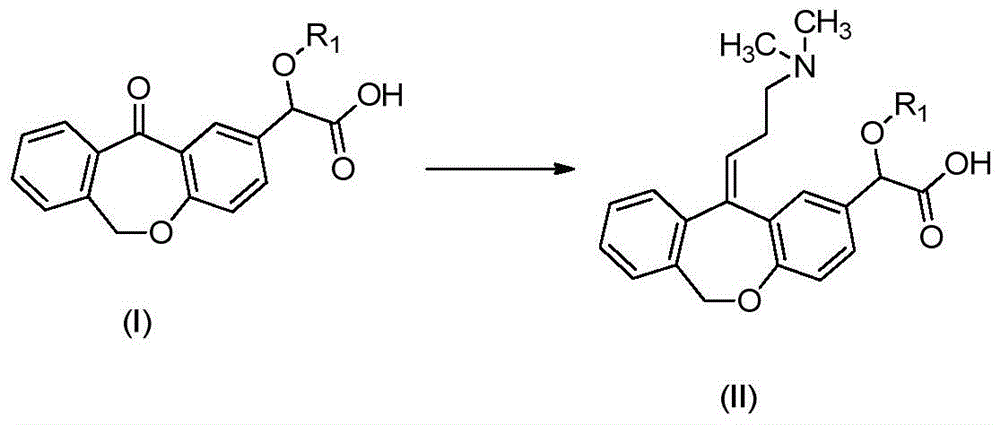
A kind of preparation method of o-hydroxy olopatadine
A technology for the protection of adjacent hydroxyl groups and hydroxyl groups, which is applied in the field of medicine, can solve problems such as no synthetic method reports, and achieve the effects of good yield, stable product quality, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Preparation of 2-hydroxy-2-(11-oxa-6,11-dihydrodibenzo[b,e]oxepin)-2-acetic acid.
[0046]
[0047] Prepare with reference to the preparation method disclosed in US4417063 Example 1-4:
[0048] Add ethyl 2-bromomethylbenzoate (100g), p-hydroxybenzaldehyde (52.5g), potassium carbonate (241g), potassium iodide (2.1g), DMF (500ml) in a 2L glass reactor (mechanical stirring), The system was heated to reflux for about 18 hours. TLC detected that the reaction was substantially complete and dropped to room temperature. After filtration, the filtrate was concentrated under reduced pressure to obtain an oil. Add 800ml of ethyl acetate to dissolve, and wash with saturated sodium carbonate solution and brine successively. The organic phase was dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated under reduced pressure to obtain an oil. Add 500ml of ethyl acetate and n-heptane mixed solution (1:12), heat to reflux, crystallize at 0-5°C, filter to ob...
Embodiment 2
[0052] Preparation of 2-benzyloxy-2-(11-oxa-6,11-dihydrodibenzo[b,e]oxepin)-2-acetic acid
[0053] 2-Hydroxy-2-(11-oxa-6,11-dihydrodibenzo[b,e]oxepin-2-acetic acid (284 g) in ethanol was added to a 2 L glass reactor (mechanically stirred) (1200ml), potassium carbonate (200g), stir at room temperature until no gas is produced, add benzyl chloride (127g), potassium iodide (8g), heat up to reflux, keep warm for 8-10 hours, TLC detection reaction is basically complete, cool to room temperature , filter.The filtrate is concentrated, add 1000ml of water, adjust to a pH value of less than 3 with 1N hydrochloric acid, add ethyl acetate for extraction, the organic phase is dried with anhydrous sodium sulfate, filter.Filter and concentrate under reduced pressure to obtain an oil, add 600ml of ethyl acetate Mix solution with n-heptane (1:10), heat to reflux, crystallize at 0-5°C, filter to obtain 2-benzyloxy-2-(11-oxa-6,11-dihydrodibenzo[b, e] oxheptin-2-acetic acid (275g). The yield is...
Embodiment 3
[0055] Preparation of (Z)-11-[3-(dimethylamino)propenyl]-6,11-dihydrodibenzo[b,e]oxepin-2-benzyloxy-2-acetic acid
[0056] Add [3-(dimethylamino)propyl]triphenylphosphine bromide hydrobromide (230g) and anhydrous tetrahydrofuran (1200ml) into a 3L glass reactor (mechanical stirring), cool to below -10°C , dropwise add butyllithium solution (2.5N, 250ml), the reaction exotherm is obvious, keep the temperature below -10°C, keep stirring for 1-2 hours, add dropwise 2-benzyloxy-2-(11-oxa- 6,11-Dihydrodibenzo[b,e]oxepin)-2-acetic acid (37.4g) in anhydrous tetrahydrofuran solution (200ml), the reaction exotherm is obvious, keep the temperature below -5 ° C, keep stirring After 1-2 hours, slowly rise to room temperature, continue stirring for 4-5 hours, and slowly add deionized water (200ml) dropwise to destroy excess butyllithium. The reaction solution was concentrated to dryness under reduced pressure, added water (1200ml) to dissolve, added methyl tert-butyl ether for extraction ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com