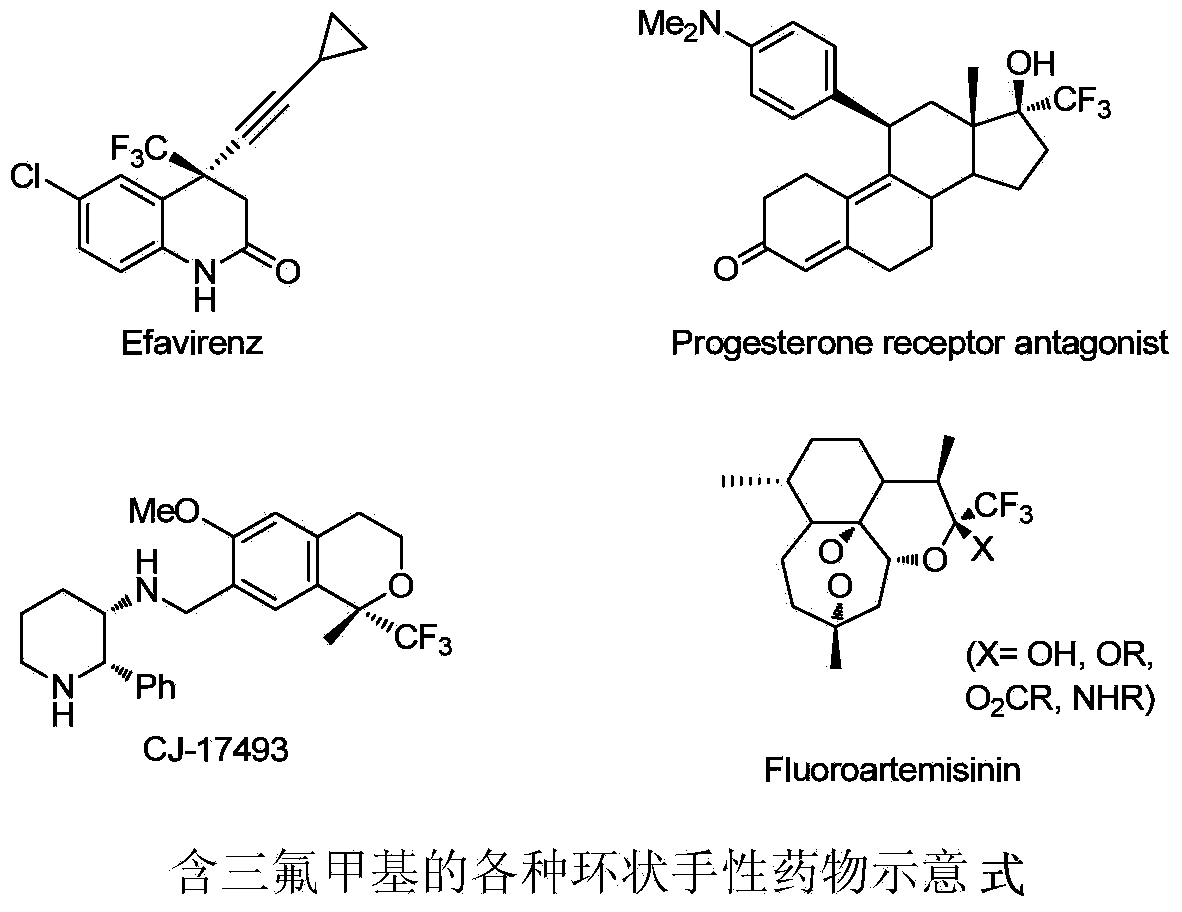
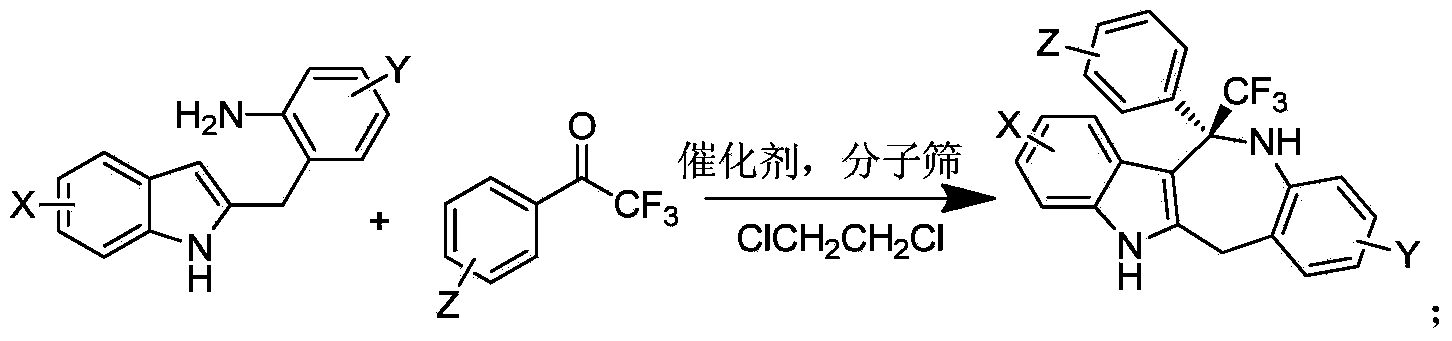
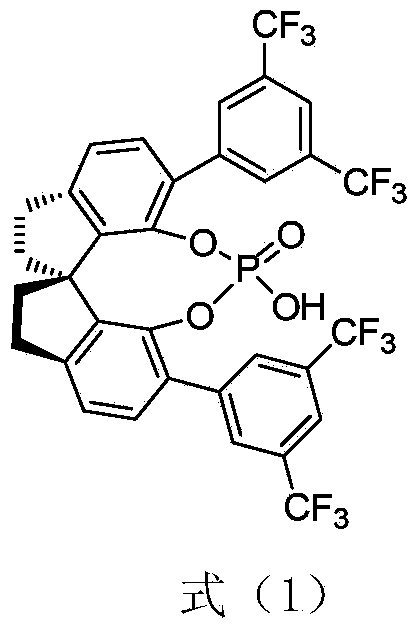
Method for chiral spirocyclic phosphoric acid catalytic synthesis of optically active benzoazepinoindole derivative
A technology of spirophosphoric acid catalysis and spirophosphoric acid catalyst, applied in the direction of organic chemistry, etc., to achieve the effects of mild reaction conditions, high enantioselectivity, and strong reaction versatility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Under nitrogen protection, add (S)-O,O'-{7,7'-[6,6'-bis-(3,5-bistrifluoromethylbenzene) of formula (1) into a reaction flask base)-1,1'-spirobisdihydroindane]} phosphoric acid catalyst catalyst (0.005mmol) and 2-o-aminobenzylindole 2 (0.1mmol), 2,2,2-trifluoroacetophenone (0.1 mmol) and 0.5 milliliters of 1,2-dichloroethane, the reaction temperature is controlled at 35 degrees. The reaction process was detected under TLC, and the reaction time was 24 hours. Directly use silica gel column chromatography after the end of the reaction to obtain optically active (S)-12-phenyl-12-trifluoromethyl-5,6,11,12-tetrahydro-benzo[6,7]azepine[ 4,3-b]indole, yield 95%. The optical purity of the product was 93% ee by HPLC.
[0021] HPLC analysis: Chiralpak AD-H (hexane / i-PrOH=95 / 5, 0.8mL / min), t R (minor)17.5min,t R (major)20.7min; [α] D 20 =-24.5 (c=0.45, CH 2 Cl 2 ); 1 H NMR (400MHz, CDCl 3 ): δ=8.08(s,1H),7.47-7.26(m,5H),7.24(s,1H),7.18(d,J=7.6Hz,1H),7.10-6.93(m,3H),6.71-...
Embodiment 2
[0025] Under nitrogen protection, add (S)-O,O'-{7,7'-[6,6'-bis-(3,5-bistrifluoromethylbenzene) of formula (1) into a reaction flask base)-1,1'-spirobisdihydroindane]} phosphoric acid catalyst catalyst (0.005mmol) and 2-o-aminobenzylindole 2 (0.1mmol), 2,2,2-trifluoroacetophenone (0.1 mmol) and 0.5 milliliters of dichloromethane, the control reaction temperature is at 35 degrees. The reaction process was detected under TLC, and the reaction time was 24 hours. Directly use silica gel column chromatography after the end of the reaction to obtain optically active (S)-12-phenyl-12-trifluoromethyl-5,6,11,12-tetrahydro-benzo[6,7]azepine[ 4,3-b]indole, yield 90%. The optical purity of the product was 89% ee by HPLC.
Embodiment 3
[0027] Under nitrogen protection, add (S)-O,O'-{7,7'-[6,6'-bis-(3,5-bistrifluoromethylbenzene) of formula (1) into a reaction flask base)-1,1'-spirobisdihydroindane]} phosphoric acid catalyst catalyst (0.005mmol) and 2-o-aminobenzylindole 2 (0.1mmol), 2,2,2-trifluoroacetophenone (0.1 mmol) and 0.5 milliliters of toluene, the control reaction temperature is at 35 degrees. The reaction process was detected under TLC, and the reaction time was 24 hours. Directly use silica gel column chromatography after the end of the reaction to obtain optically active (S)-12-phenyl-12-trifluoromethyl-5,6,11,12-tetrahydro-benzo[6,7]azepine[ 4,3-b]indole, 85% yield. The optical purity of the product was 89% ee by HPLC.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com