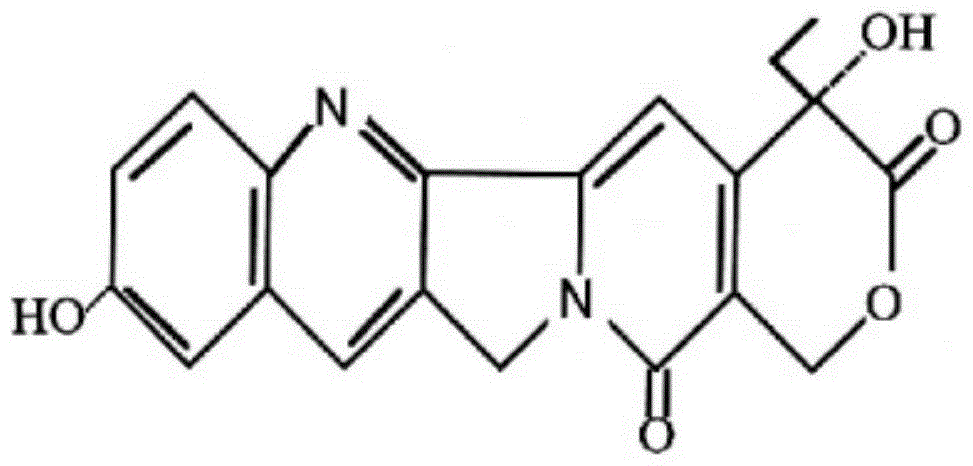
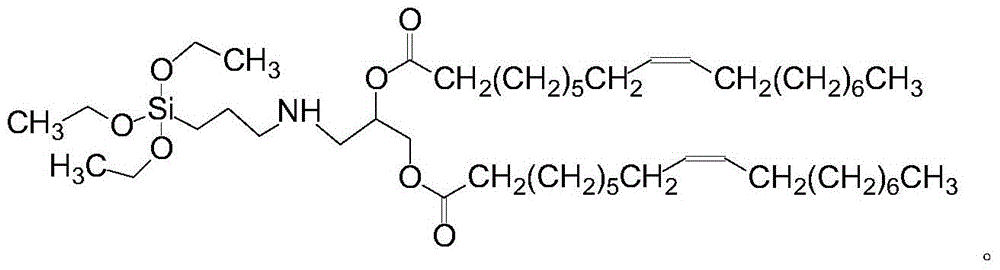
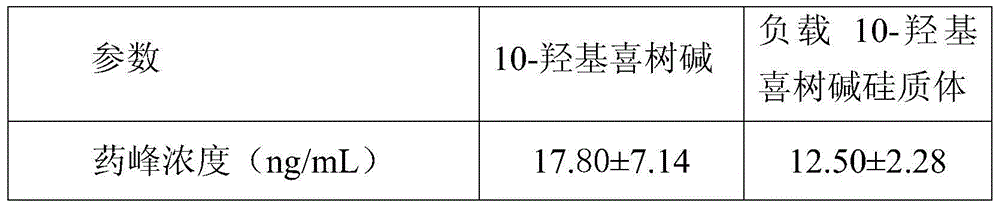
Silica body loaded with 10-hydroxycamptothecin and its preparation method
A technology of hydroxycamptothecin and siliceous body, which is applied in the fields of medicinal chemistry and therapeutics, can solve the problems of drug loading not exceeding 2%, low siliceous body encapsulation rate, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1 Synthesis of Silicon-containing Lipid Molecules
[0028] Add 2.3 grams of 1,3-oleic acid glyceride into dry dichloromethane, and after fully dissolving, continue to add 1 gram of aminopropyltriethoxysilane and 5 grams of 1-(3-dimethylaminopropyl base)-3-ethylcarbodiimide hydrochloride, and the reaction system was stirred at 25°C for 4 hours. The reaction product was rotary evaporated under vacuum conditions to obtain a crude product, which was purified by column chromatography and then obtained by rotary evaporation to obtain silicon-containing lipid molecules with a yield of 50%.
[0029] 1 HNMR (400MHz, CDCl 3 ,TMS,ppm):δ0.59(2H,t),0.85(6H,t),1.21(9H,t),1.22-1.26(54H,m),1.489-1.64(6H,m),2.59(2H ,t),3.19-3.30(2H,m),3.49-3.53(2H,t),3.63-3.66(4H,t),3.68-3.86(6H,m),3.95-4.10(4H,t)6.40(1H ,s).
Embodiment 2
[0030] Embodiment 2 Synthesis of Silicon-containing Lipid Molecules
[0031] Add 3.4 grams of 1,3-oleic acid glyceride into dry dichloromethane, and after fully dissolving, continue to add 0.8 grams of aminopropyltriethoxysilane and 6.2 grams of 1-(3-dimethylaminopropyl base)-3-ethylcarbodiimide hydrochloride, and the reaction system was stirred at 27.5°C for 3.8 hours. The reaction product was rotary evaporated under vacuum to obtain a crude product, which was purified by column chromatography and then obtained by rotary evaporation to obtain silicon-containing lipid molecules with a yield of 55%.
[0032] 1 HNMR (400MHz, CDCl 3 ,TMS,ppm):δ0.59(2H,t),0.85(6H,t),1.21(9H,t),1.22-1.26(54H,m),1.489-1.64(6H,m),2.59(2H ,t),3.19-3.30(2H,m),3.49-3.53(2H,t),3.63-3.66(4H,t),3.68-3.86(6H,m),3.95-4.10(4H,t)6.40(1H ,s).
Embodiment 3
[0033] The synthesis of embodiment 3 silicon-containing lipid molecules
[0034] Add 1.5 grams of 1,3-oleic acid glyceride to dry dichloromethane. After fully dissolving, continue to add 0.78 grams of aminopropyltriethoxysilane and 4.4 grams of 1-(3-dimethylaminopropyl base)-3-ethylcarbodiimide hydrochloride, and the reaction system was stirred at 23°C for 6 hours. The reaction product was rotary evaporated under vacuum to obtain a crude product, which was purified by column chromatography and then obtained by rotary evaporation to obtain silicon-containing lipid molecules with a yield of 58.5%.
[0035] 1 HNMR (400MHz, CDCl 3 ,TMS,ppm):δ0.59(2H,t),0.85(6H,t),1.21(9H,t),1.22-1.26(54H,m),1.489-1.64(6H,m),2.59(2H ,t),3.19-3.30(2H,m),3.49-3.53(2H,t),3.63-3.66(4H,t),3.68-3.86(6H,m),3.95-4.10(4H,t)6.40(1H ,s).
PUM
| Property | Measurement | Unit |
|---|---|---|
| encapsulation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com