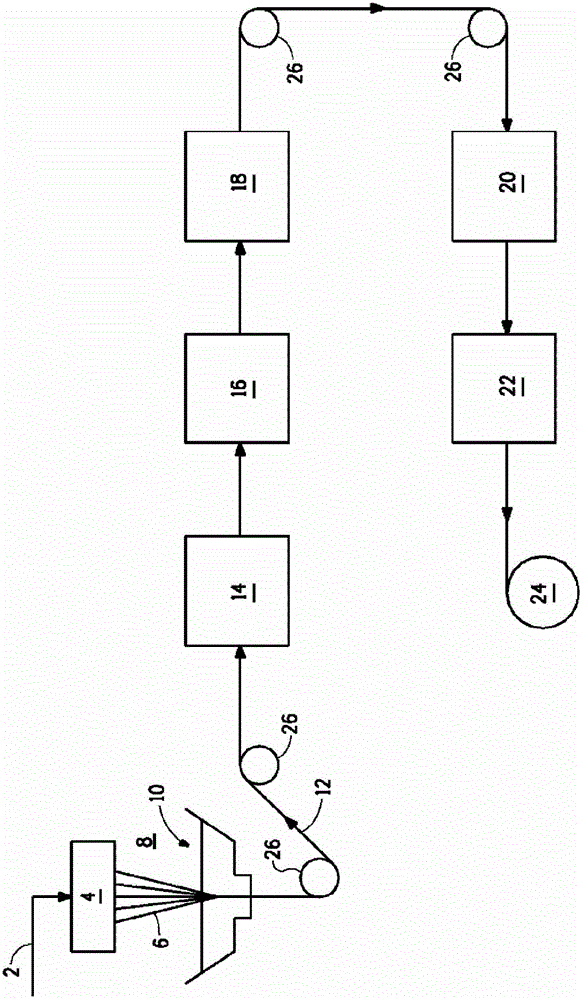
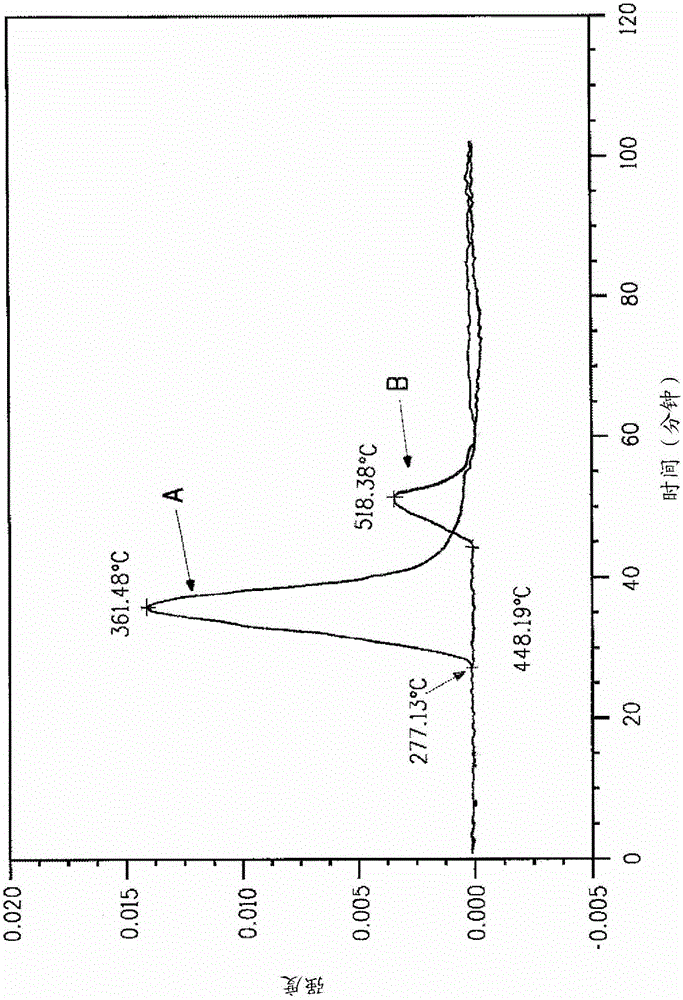
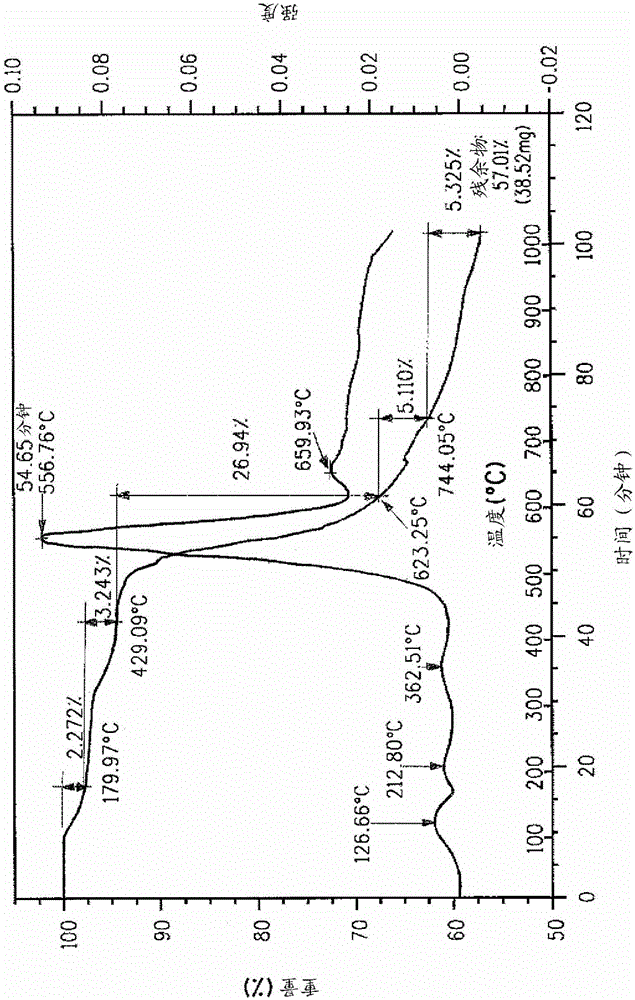
Method for removing sulfur from fiber using an aqueous acid
An aqueous acid, fiber technology, applied in the field of using aqueous acid to remove sulfur from fibers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0079] The FM130D Littleford reactor was loaded with calcium chloride (CaCl 2 ) of N-methyl-2-pyrrolidone (NMP) solvent. Appropriate amounts of monomers 5(6)-amino-2-(p-aminophenyl)benzimidazole (DAPBI) and terephthaloyl dichloride (TCL) were then added to the reactor and reacted to form oligomers. To this mixture, appropriate amounts of p-phenylenediamine (PPD) and TCL were added to form the final copolymer crumb. The crumbs were milled into smaller particles and then washed first with sodium hydroxide solution to neutralize reaction by-products and then with water to remove NMP. The polymer was then recovered, dried and its measured intrinsic viscosity is summarized in Table 1.
[0080] Table 1
[0081] project
[0082] P1-3
example 2
[0084] The FM130D Littleford reactor was loaded with calcium chloride (CaCl 2 ) of N-methyl-2-pyrrolidone (NMP) solvent. Then, an appropriate amount of monomer 5(6)-amino-2-(p-aminophenyl 1 benzimidazole (DAPBI), PPD, and part of terephthaloyl dichloride (TCL) was added to the reactor and reacted to form Oligomers. To this mixture, an appropriate amount of TCL is added to form the final copolymer crumb. The crumb is ground into smaller particles and then first washed with sodium hydroxide solution to neutralize the reaction by-products, then water Washing to remove NMP.The polymer was then recovered, dried and its measured intrinsic viscosity is summarized in Table 2.
[0085] Table 2
[0086] project
DAPBI / PPD molar ratio
Intrinsic viscosity (dl / g)
P2-1
40 / 60
7.00
P2-2
50 / 50
6.39
P2-3
75 / 25
3.98
[0087] fiber example
[0088] In the following examples, solution spinning of the copolymer in concentr...
example 2 and comparative example C
[0106] A concentrated sulfuric acid solution of the polymer at a solids concentration of 22% by weight was formed using a neutralized copolymer made of TCL and DAPBI / PPD with a diamine molar ratio of 70 / 30. The copolymer solution was spun through a spinneret having 270 holes to produce a nominal linear density of 1.75 denier / filament. The yarn was coagulated and washed to 3.00% by weight sulfur.
[0107] The yarn was then washed continuously at 24 m / min in 9 wash cabinets. The second box used the HCl wash solution as given in Table 6, all other boxes used water. The first wash box uses 10 advancing yarn coils through the wash spray and applicator, however, the remaining 8 wash boxes use 20 advancing yarn coils through the wash spray and applicator. All wash modules were operated at 60°C. The yarn was dried in-line under a temperature ramp of 130°C to 205°C along the length of the oven at a tension of 0.5 g / denier. The yarn was then heat treated using a maximum temperature ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com