Preparation method of octahedral Mn3O4 nanoparticles
A nanoparticle and octahedron technology, which is applied in the field of synthesis of nanomaterials for chemical power sources, can solve the problems of few reports on air electrode catalysts for aluminum-air batteries, and achieve excellent oxygen reduction catalytic performance, low cost, and simple process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
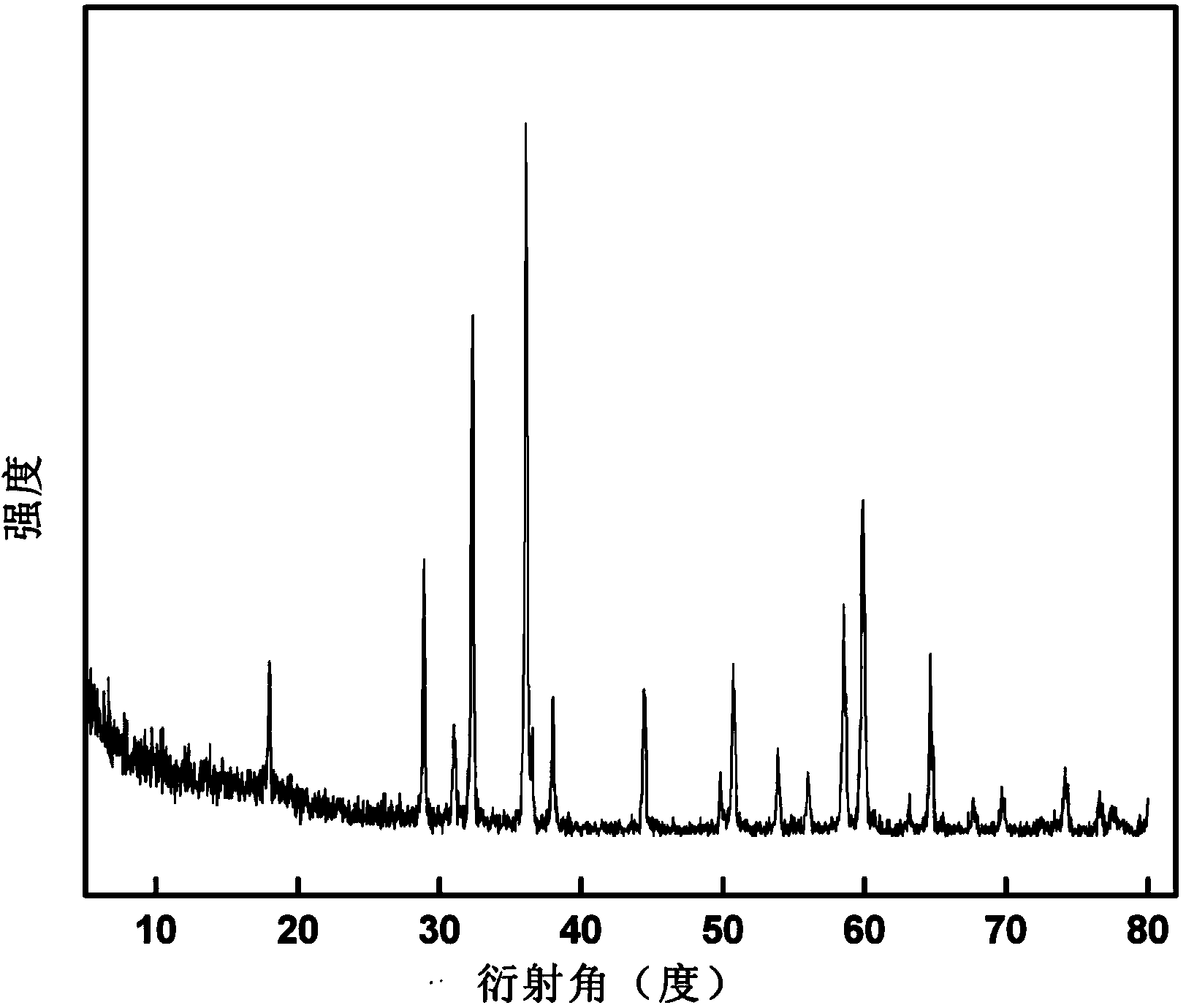
[0032] Weigh 0.4g of potassium permanganate for later use, accurately measure 20ml of N-N-dimethylformamide (DMF) and 50ml of deionized water, mix the two evenly, and then add potassium permanganate. After being stirred evenly, it was transferred to a high-pressure reactor with a volume of 100 ml and lined with polytetrafluoroethylene, and the reaction temperature was set at 140° C., and the reaction time was 12 hours. After the reaction was completed, it was naturally cooled to room temperature and reduced Suction filtration, washing, vacuum drying at 60°C for 24 hours, and finally grinding to obtain the product. The resulting product is characterized by XRD, and its XRD pattern is shown in figure 1 , showing that the product prepared by this example is pure phase Mn 3 o 4 . SEM observation of the product ( figure 2 ) shows that the obtained product is a regular octahedron with a particle size of 400-500nm.
[0033] The prepared target material, activated carbon, acetyl...
Embodiment 2
[0035] Weigh 0.4g of potassium permanganate for later use, accurately measure 35ml of N-N-dimethylformamide (DMF) and 35ml of deionized water, mix the two evenly, and then add potassium permanganate. After being stirred evenly, it was transferred to a high-pressure reactor with a volume of 100 ml and lined with polytetrafluoroethylene, and the reaction temperature was set at 140° C., and the reaction time was 12 hours. After the reaction was completed, it was naturally cooled to room temperature and reduced Suction filtration, washing, vacuum drying at 60°C for 24 hours, and finally grinding to obtain the product. The resulting product is characterized by XRD, and the result shows that the product prepared by this embodiment is a pure phase Mn 3 o 4 . The scanning electron microscope observation of the product shows that the obtained product is a regular octahedron with a regular shape and a particle size of 400-500nm.
Embodiment 3
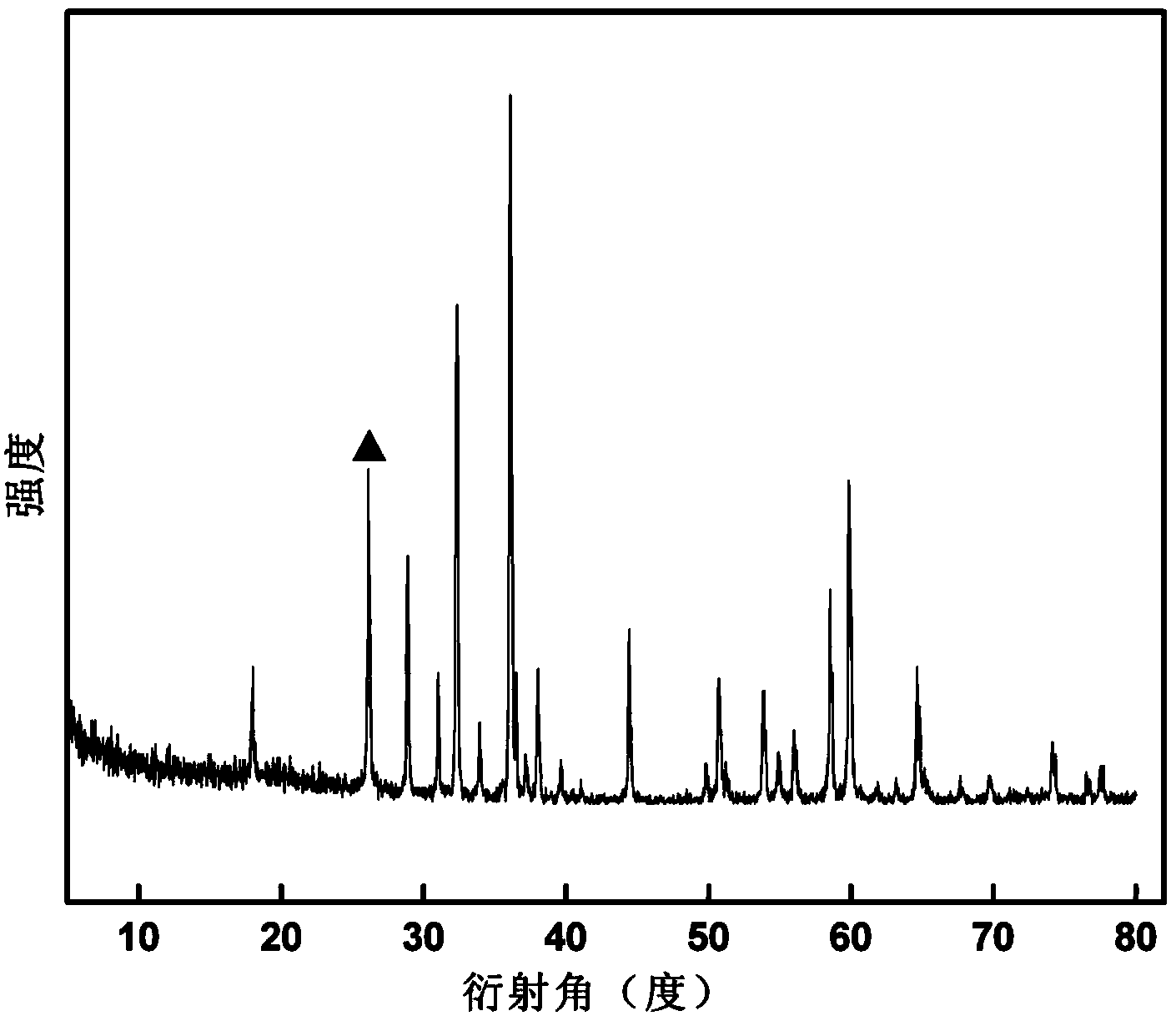
[0037] Weigh 0.4g of potassium permanganate for later use, accurately measure 20ml of N-N-dimethylformamide (DMF) and 50ml of deionized water, mix the two evenly, and then add potassium permanganate. After stirring evenly, transfer it to a high-pressure reactor with a volume of 100 ml and lined with polytetrafluoroethylene, set the reaction temperature to 120 ° C, and the reaction time to be 12 hours. After the reaction is completed, naturally cool to room temperature and reduce Suction filtration, washing, vacuum drying at 60°C for 24 hours, and finally grinding to obtain the product. The resulting product is characterized by XRD, and its XRD spectrum is shown in image 3 , the results show that the main phase of the product prepared in this example is Mn 3 o 4 , the secondary phase is MnOOH. SEM observation of the product ( Figure 4) shows that the obtained product is mainly regular octahedron with a small amount of rod-like morphology.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com