Sesquiterpene compound with antitumor activity and preparation method thereof
A technology of sesquiterpenoids and compounds, applied in the field of sesquiterpenoids and their preparation, can solve problems such as drugs that have not yet been seen, and achieve the effects of novel structure, simple preparation method, and strong anti-tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
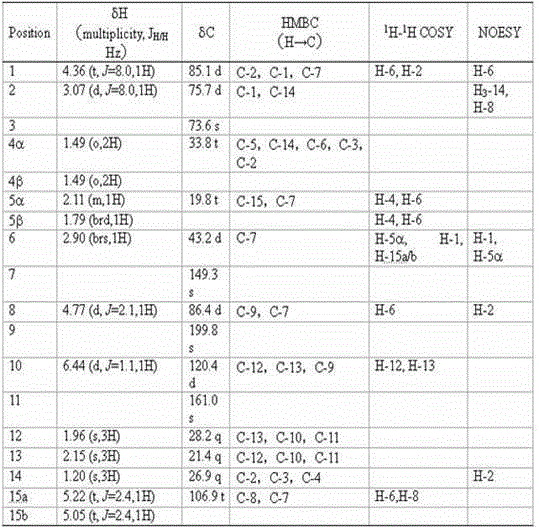
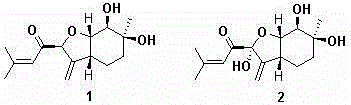
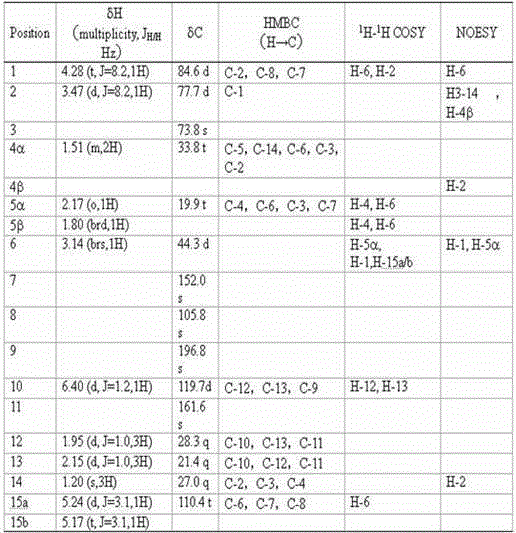
[0022] Prepare solid PDA medium (15 L per liter of water) and divide into 750 90 mm glass petri dishes, each containing about 20 mL of medium, insert activated wild abalone mushroom ( Pleurotus cystidiosus ) ZYB2013 (preserved in the China Center for Type Culture Collection on June 15, 2014, with the preservation number CCTCC M 2014256) mycelium block, cultured in a constant temperature incubator at 28 °C for 43 days. After cultivation, chop wild abalone mushroom mycelium together with the culture medium, put it in a faucet bottle, add organic solvent (ethyl acetate: methanol: acetic acid volume ratio = 80:15:5) for leaching 6 times, and collect the extract , concentrated under reduced pressure at 40°C to a paste, extracted 6 times with pure water and ethyl acetate 1:1, dehydrated the ethyl acetate phase with anhydrous sodium sulfate, concentrated under reduced pressure at 40°C to a paste, The ethyl acetate crude extract (4.4057 g) was obtained.
[0023] The 4.4057 g ethyl ac...
Embodiment 2
[0034] The inhibitory effects of the compounds on prostate cancer cells DU-145, C42B and LNCaP were determined by MTT method. The cultured prostate cancer cells DU-145, C42B and LNCaP were made into a single cell suspension, counted with a cell plate and diluted to a cell concentration of 6×10 4 individual / mL. Cells were seeded in 96-well plates, 80 μL per well. In addition, 2 wells without cells and only 80 μL of culture medium [Dulbecco’s modified Eagle’s media (DMEM, Gibco, USA) + 10% calf serum] were set as blank control wells for instrument zeroing. Set at 37°C, 5% CO 2 Incubate in an incubator for 24 h, and then add 20 μL of the sample diluted with culture medium. At the same time, add 20 μL of cisplatin to the positive control wells, and add 20 μL of culture solution to the negative control wells and blank control wells. Continue to culture for 72 hours, and add 10 μL of 5 mg / mL MTT to each well. React at 37°C for 3 h, add 100 μL of 10% SDS-0.01mol / L HCl to each we...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com